Stabilization of Cu(i) for binding and calorimetric measurements in aqueous solution†
Abstract
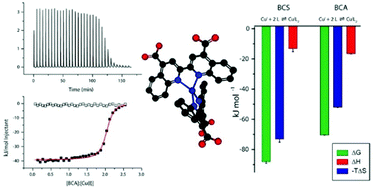
Conditions have been developed for the comproportionation reaction of Cu2+ and copper metal to prepare aqueous solutions of Cu+ that are stabilized from disproportionation by MeCN and other Cu+-stabilizing ligands. These solutions were then used in ITC measurements to quantify the thermodynamics of formation of a set of Cu+ complexes (CuI(MeCN)3+, CuIMe6Trien+, CuI(BCA)23−, CuI(BCS)23−), which have stabilities ranging over 15 orders of magnitude, for their use in binding and calorimetric measurements of Cu+ interaction with proteins and other biological macromolecules. These complexes were then used to determine the stability and thermodynamics of formation of a 1 : 1 complex of Cu+ with the biologically important tri-peptide glutathione, GSH. These results identify Me6Trien as an attractive Cu+-stabilizing ligand for calorimetric experiments, and suggest that caution should be used with MeCN to stabilize Cu+ due to its potential for participating in unquantifiable ternary interactions.

 Please wait while we load your content...
Please wait while we load your content...