Promiscuous hydroxylases for the functionalization of polycyclic tetramate macrolactams – conversion of ikarugamycin to butremycin†‡
Abstract
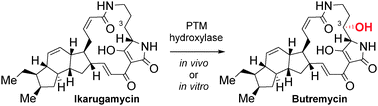
Polycyclic tetramate macrolactams (PTMs) are a structurally, biomedically and biosynthetically intriguing class of bacterial metabolites. By combining parts of the machineries of different PTM biosynthetic pathways, we demonstrate for the first time the substrate promiscuity of a class of PTM tailoring enzymes, thereby facilitating the (bio)synthesis of butremycin.
- This article is part of the themed collection: 2015 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...