Analysis of the residual alignment of a paramagnetic multiheme cytochrome by NMR†
Abstract
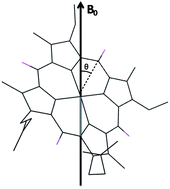
Residual dipolar couplings measured by NMR spectroscopy reveal that the rhombicity of the electronic structure of low-spin paramagnetic hemes determines their relative contribution to the preferential orientation of a protein with multiple hemes when placed in a strong magnetic field.
- This article is part of the themed collection: Biological oxidation reactions: mechanisms and design of new catalysts

 Please wait while we load your content...
Please wait while we load your content...