Room temperature decarboxylative trifluoromethylation of α,β-unsaturated carboxylic acids by photoredox catalysis†
Abstract
A visible-light-induced decarboxylative trifluoromethylation of α,β-unsaturated carboxylic acids, which uses the Togni reagent as the CF3 source is disclosed. The corresponding trifluoromethylated alkenes were obtained in moderate to high yields with excellent functional group tolerance at ambient temperature. Preliminary mechanistic analyses suggest a radical-type mechanism.
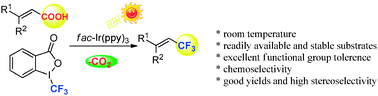

 Please wait while we load your content...
Please wait while we load your content...