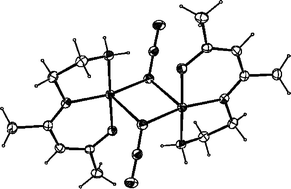
Three new basal–apical, μ2-1,1-azide bridged complexes, [CuL1(N3)]2
(1), [CuL2(N3)]2
(2) and [CuL3(N3)]2
(3) with very similar tridentate Schiff base blocking ligands [L1
=
N-(3-aminopropyl)salicylaldimine, L2
= 7-amino-4-methyl-5-azahept-3-en-2-one and L3
= 8-amino-4-methyl-5-azaoct-3-en-2-one) have been synthesised and their molecular structures determined by X-ray crystallography. In complex 1, there is no inter-dimer H-bonding. However, complexes 2 and 3 form two different supramolecular structures in which the dinuclear entities are linked by strong H-bonds giving one-dimensional systems. Variable-temperature (300–2 K) magnetic susceptibility measurements and magnetization measurements at 2 K reveal that complexes 1 and 2 have antiferromagnetic coupling while 3 has ferromagnetic coupling which is also confirmed by EPR spectra at 4–300 K. Magnetostructural correlations have been made taking into consideration both the azido bridging ligands and the existence of intermolecular hydrogen bonds in complexes 2 and 3.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...