Electrocatalytic hydrogenation of quinoxaline using CoO/NF in organic molecular redox flow batteries
Xin
Zheng
a,
Bowen
Chen
b,
Hanyu
Li
a,
Fangcheng
Qiu
a,
Xue
Han
b,
Shaowen
Tan
b,
Siyi
Chen
b and
Shengping
Wang
 *b
*b
aElectric Power Research Institute, Yunnan Power Grid Co., Ltd, Kunming 650220, China
bFaculty of Material Science and Chemistry, China University of Geosciences, Wuhan 430074, China. E-mail: spwang@cug.edu.cn
First published on 19th November 2025
Abstract
To advance the application of liquid organic hydrogen carriers (LOHCs) in flow batteries, the mechanism and performance of CoO/NF for the electrocatalytic hydrogenation (ECH) of quinoxaline—prepared by a low-melting-point ionic liquid electrodeposition method—are systematically investigated. The quinoxaline/tetrahydroquinoxaline conversion reaction catalyzed with CoO/NF results in superior ECH activity with low charge transfer impedance (1.624 ohm) and a Tafel slope of 103 mV dec−1; the efficiency of quinoxaline conversion is 99.84%, and the selectivity of tetrahydroquinoxaline formation is 98.73%. The hydrogen in the hydrogenation reaction comes from water, and the active hydrogen atoms (H*) generated on the cobalt surface via the Volmer step are the key intermediates. The electrocatalyzed quinoxaline/tetrahydroquinoxaline reaction is an efficient system for hydrogen storage in flow batteries, providing a scientific basis for hydrogen energy storage and conversion in LOHC-based flow batteries.
1 Introduction
In recent years, liquid organic hydrogen carriers (LOHCs) have shown great potential as efficient and safe hydrogen storage and transportation solutions.1,2 However, to achieve large-scale industrialization, the core technical challenge lies in significantly reducing the harshness of hydrogenation reaction conditions and effectively controlling costs. This urgently requires breakthrough improvements in hydrogen storage technology. Traditional thermodynamic catalytic hydrogenation pathways typically face stringent operational requirements, such as high temperatures and pressures and complex external hydrogen supply conditions.1,3,4 Quinoline hydrogenation with a yield of 94% was achieved using manganese under mild conditions.5 This achievement represents a significant step forward in reducing the harshness of reaction conditions. However, this method faces a critical bottleneck: recovering and reusing the catalyst is difficult, which is a significant disadvantage in industrial-scale continuous production and limits its practical application potential.Unlike thermodynamic hydrogen storage, electrocatalytic hydrogenation (ECH) has become popular for LOHC hydrogen storage because of its milder reaction conditions and ability to achieve more precise ECH by regulating the potential.6–9 Electrochemical methods directly utilize active hydrogen atoms (H*) generated from water by electrolysis for hydrogenation,10 eliminating the need for an external high-pressure hydrogen gas source and significantly simplifying the system. Researchers have instead opted for liquid hydrogen sources (such as water or alcohols) as electron donors.11 Catalysts under alkaline conditions, such as MoNi4/NF,12 CuCo2O4/NF,13 P-WO3/NF,14 Pd/NF,15 and NiCoP/NF,16 can achieve high-selectivity hydrogenation of LOHCs under ambient temperature and pressure, with selectivities exceeding 90%.
Electrodeposition is an effective method for synthesizing catalysts,17–20 where low-melting-point ionic liquids are used as the electrolyte for electrodeposition,21–24 which increases the faradaic efficiency and reduces the number of hydrogen evolution reactions (HERs). More importantly, it avoids the mechanical damage to the deposited layer caused by bubbles generated during the HER. In this study, CoO/NF was electrodeposited in a low-melting-point ionic liquid,25,26 and the ECH of quinoxaline was investigated.
2 Experimental section
2.1 Preparation of CoO/NF
2.2 Material characterization
Scanning electron microscopy (SEM) was performed using a Hitachi SU8010 system. The morphology and size of the samples were observed, as were the changes in morphology and stability before and after cycling. The elemental distribution was determined using energy-dispersive X-ray spectroscopy (EDS), and qualitative and quantitative analyses of the elemental composition of the catalyst electrode surface were conducted. The material structure was determined using a Bruker D8-Focus X-ray powder diffraction (XRD) instrument employing a Cu Kα-target (λ = 0.1542 nm), with a scanning range of 5–90° and a scanning speed of 10° min−1. This characterized the change in the crystal structure of the catalyst electrodes before and after cycling and structural stability. X-ray photoelectron spectroscopy (XPS) tests were conducted using a Thermo Scientific Escalab instrument to analyze the composition of the samples and the valence states of the metal elements, as well as changes in the metal valence states before and after catalysis. All the peaks were corrected using the C 1s spectrum with a binding energy of 284.8 eV.The contents of quinoxaline (QXL) and tetrahydroquinoxaline (THQXL) in the electrolyte were determined using Pano A60 gas chromatography (GC). The gas chromatograph was equipped with an AB-1 column (30 m × 0.32 mm × 0.25 µm), with an injection temperature of 300 °C, a detector temperature of 300 °C, and a column oven temperature of 300 °C. The temperature was increased from 100 °C to 300 °C at a rate of 10 °C min−1 and held for 5 min.
2.3 Electrochemical device for ECH
The preparation of the electrolyte and the assembly of the electrolytic cell were carried out in an argon-filled glove box at 25 °C. After preparation, the electrolyte was flushed with argon for 1 h. Electrolyte 1 was 1.0 M KOH. Electrolyte 2 was 1.0 M KOH with 15 mM quinoxaline. Electrolyte 3 was 1.0 M KOH with 51 mM t-butyl alcohol (t-BuOH). Electrolyte 4 was 1.0 M KOH with 15 mM quinoxaline and 51 mM t-BuOH. In the H-type electrolytic cell separated by a Nafion 117 membrane, the catalyst was used as the working electrode, a Hg/HgO electrode was used as the reference electrode, a Pt electrode (1 cm × 1 cm) was used as the counter electrode, and the electrolyte volume on both the working and counter electrode sides was 35 mL.2.4 Electrochemical measurements
All the electrochemical tests were conducted in a glove box filled with argon gas at 25 °C, and all the electrochemical potentials were converted to a reversible hydrogen electrode (RHE) with eqn (1).| ERHE (V) = E(Hg/HgO) + 0.0591 × pH + E0(Hg/HgO) = E(Hg/HgO) + 0.059 × pH + 0.098 | (1) |
The catalyst was used as the working electrode, with Electrolyte 2 used as the electrolyte on the working electrode side and Electrolyte 1 used as the counter electrode electrolyte, and LSV testing was performed at 5 mV s−1 within the potential range from the OCP to −0.424 V (vs. RHE) to obtain the ECH current density at different potentials.
The catalyst was used as the working electrode, with Electrolyte 2 used as the electrolyte on the working electrode side and Electrolyte 1 used as the electrolyte on the counter electrode side, and LSV tests were conducted at 1 mV s−1 within the potential range from the OCP to −0.424 V (vs. RHE). After LSV testing was completed, the Tafel slope was calculated by linearly fitting the selected region data according to the Tafel equation (eqn (2)).
η = a + b × log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j j | (2) |
The catalyst electrodes were used as the working electrodes, with Electrolyte 1 used as the electrolyte on the working electrode side and Electrolyte 1 used as the electrolyte on the counter electrode side. Activation was performed by scanning for 500 cycles at 100 mV s−1 with a potential range of OCP ± 0.05 V. After activation, CV tests were conducted at 20, 40, 60, 80, and 100 mV s−1 to determine the double-layer capacitance (Cdl), and the electrochemically active surface area (ECSA) was calculated with eqn (3).
| ECSA = Cdl/Cs | (3) |
To assess the long-term stability of the catalyst, four chronoamperometry tests were conducted for 2 h at a constant potential of −0.3 V (vs. RHE). The hydrogenation products were detected six times using GC, and the conversion rate and selectivity rate during long-term cycling were calculated.
 | (4) |
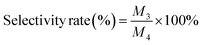 | (5) |
3 Results and discussion
3.1 Physical characterization
The diffraction peaks of CoO/NF at 44.39°, 47.55°, and 75.93° corresponded to the (002), (101), and (110) planes of Co (PDF #05-0727), respectively, and the weak peaks at 41.88° and 61.52° corresponded to the (200) and (020) planes of CoO (PDF #43-1004), respectively (Fig. 1c). The sample met the expected preparation requirements. The CoO/NF prepared by electrodepositing Co onto NF exhibits densely grown nanosheets on the original NF substrate (Fig. 1d and e), significantly increasing the active surface area of the catalyst. This provides more active sites for the adsorption of quinoxaline and H*, thereby increasing the ECH activity. The elements of CoO/NF are uniformly distributed (Fig. 1f–i). The presence of a small amount of Ni indicates that part of the NF substrate remains exposed, which is advantageous for adsorbing the H* generated by water splitting, preventing their combination from forming hydrogen gas, and improving faradaic efficiency. | ||
| Fig. 1 (a) Co 2p and (b) O 1s XPS spectra and (c) XRD pattern of CoO/NF. SEM and EDS images of (d) NF and (e–i) CoO/NF. | ||
For the Co 2p XPS spectrum (Fig. 1a), the peaks at 780.41 and 796.35 eV corresponded to Co3+ 2p3/2 and Co3+ 2p1/2, respectively,17,28 and the peaks at 783.11 and 798.28 eV were attributed to Co2+ 2p3/2 and Co2+ 2p1/2, respectively.29 Additionally, two satellite peaks at 802.31 and 786.45 eV were identified as satellite peaks of Co2+.17 Notably, a weak but distinct characteristic peak was detected at 778.60 eV, which fell within the range of standard binding energies for metallic cobalt (Co0) 2p3/2,30 indicating the presence of partially unoxidized metallic cobalt during the electroplating process. These findings are corroborated by the metallic cobalt diffraction peak (44.39°) from the XRD results. For the O 1s XPS spectrum (Fig. 1b), the three peaks at 529.13, 530.89, and 532.73 eV were labeled OL, OV and OC, respectively. OL was attributed to typical lattice oxygen,31 which provided the structural framework for the Co–O bonds, maintained catalyst stability, and served as an electron transport channel that participated in redox reactions. OV was associated with a large number of low-oxygen-coordinated defects,32 where oxygen defects served as the primary site for hydrogen bond formation. Its peak contribution of 32.71% indicated strong hydrophilicity and water activation capability. OC was attributed to adsorbed oxygen resulting from the physical and chemical adsorption of water on the surface.
3.2 Electrochemical testing
The ECH of quinoxaline as an electrocatalyst was investigated in Electrolytes 1 and 2. The HER current density of NF in Electrolyte 1 is higher than the ECH current density in Electrolyte 2 (Fig. 2a), indicating that it inherently possesses strong HER activity but does not exhibit significant ECH effects on quinoxaline. The current density of CoO/NF in Electrolyte 1 is significantly higher than that of NF, indicating its excellent catalytic activity for the HER.33 Additionally, the reaction current in Electrolyte 2 significantly increases after quinoxaline is added because of the coordination interaction between cobalt ions, which act as Lewis acids, and the pyridine nitrogen atoms in quinoxaline molecules, which act as Lewis bases,34 resulting in an attractive effect on quinoxaline, a significant increase in the ECH current and the excellent ECH activity.CoO/NF in Electrolyte 2 exhibited a low charge transfer resistance (1.624 ohms) (Fig. 2b and Table 1), primarily attributed to the high specific surface area provided by the CoO nanosheet structure constructed on the NF substrate, which effectively expanded the electrochemically active interface. Concurrently, the Co2+ active sites and their associated oxygen vacancies exerted synergistic catalytic effects. Oxygen vacancies, as strong adsorption sites, polarized water molecules, significantly lowered the dissociation energy barrier of O–H bonds and thereby accelerated the kinetics of the Volmer step. Furthermore, this synergy optimized the catalyst's interfacial electronic structure, enhancing charge transport efficiency. The corresponding Tafel slope of 103 mV dec−1 (Fig. 2f) further confirmed that this electrode exhibits faster interfacial reaction kinetics and more efficient electrochemical reduction processes at the same overpotential. No obvious redox peaks were observed from the CV curves of NF in Electrolytes 1 and 2 (Fig. 2d), indicating that no changes in the valence state occurred in the nickel matrix within this potential window. The reduction peak of quinoxaline might have been masked by strong competition from the HER, thus explaining its potential absence in the CV curves. There was an oxidation peak at 0.28 V (vs. RHE) and a reduction peak at 0.02 V (vs. RHE) in the CV curves of CoO/NF in Electrolyte 1 (Fig. 2e); these peaks were the redox peaks of cobalt. In the CV curve of CoO/NF in Electrolyte 2, the oxidation peak of cobalt decreased in intensity, and the reduction peak disappeared. This finding is because quinoxaline hydrogenation consumes the reduction charge of the catalyst, inhibiting the reduction of Co3+ and thereby demonstrating that the catalytic sites participate in the hydrogenation reaction.
| Electrochemical parameters | NF | CoO/NF |
|---|---|---|
| HER current density (mA cm−2) from LSV at −0.3 V (vs. RHE) | −54 | −114 |
| ECH current density (mA cm−2) from LSV at −0.3 V (vs. RHE) | −62 | −166 |
| Charge transfer resistance (ohm) from EIS at −0.3 V (vs. RHE) | 3.625 | 1.624 |
| ECSA (cm2) | 16.25 | 179.25 |
| Tafel slope (mV dec−1) | 199 | 103 |
| Conversion rate after chronoamperometry at −0.3 V (vs. RHE) for 4 h (%) | 74.9 | 99.84 |
| Selectivity rate after chronoamperometry at −0.3 V (vs. RHE) for 4 h (%) | 0 | 98.73 |
On the basis of the CV curves, the electrochemically active surface area (ECSA) of the electrocatalyst was evaluated by calculating the double-layer capacitance (Cdl) value. The ECSAs of NF and CoO/NF were 16.25 and 179.25 cm2, respectively (Fig. 2c and Table 1). The large ECSA of CoO/NF was attributed primarily to the dense growth of nanosheets on the NF surface, which significantly increased the active surface area. This structure provided a larger specific surface area and a richer crystal composition, enabling efficient active centers to be easily exposed and exhibit excellent intrinsic activity.
To investigate the relationship between the faradaic efficiency and potential, chronoamperometry was conducted at different potentials for 4 h.14,15 Conversion of a significant amount of quinoxaline was achieved at −0.15 V (vs. RHE) (Fig. 3a). However, owing to the insufficient driving force for the addition reaction, high conversion rates and faradaic efficiency could not be obtained, resulting in quinoxaline being hydrogenated only to dihydroquinoxaline and being unable to undergo further hydrogenation. As the overpotential further increased, the selectivity rate for tetrahydroquinoxaline gradually increased. The quinoxaline conversion rate at −0.3 V (vs. RHE) reached 99.84% (Table 1), with an extremely high selectivity rate for tetrahydroquinoxaline (98.73%). The conversion rates and selectivity rate at −0.35 V (vs. RHE) no longer significantly increased and tended to stabilize.
Owing to the consumption of quinoxaline, the ECH current density for CoO/NF continuously decreased during the reaction. When quinoxaline was largely consumed, the current stabilized, with the residual current primarily attributed to the HER. In stark contrast, NF itself lacks the ECH activity of quinoxaline, and its current density remained constant throughout the reaction. GC analysis further confirmed the aforementioned activity differences. CoO/NF successfully achieved the hydrogenation of quinoxaline to produce the target product tetrahydroquinoxaline. However, tetrahydroquinoxaline was not detected in the products catalyzed by NF, which conclusively demonstrates that NF lacks intrinsic catalytic activity for the ECH of quinoxaline. After 4 h of reaction with NF (Fig. 3b), the conversion rate for quinoxaline was 74.9%, but no tetrahydroquinoxaline was produced. This conversion was likely attributed primarily to nonhydrogenation pathways. In contrast, CoO/NF exhibited excellent bifunctional catalytic performance,3 combining HER activity with highly efficient quinoxaline hydrogenation activity, achieving nearly complete conversion of quinoxaline (99.84%) and an extremely high rate of tetrahydroquinoxaline selectivity (98.73%) (Table S1). The electrocatalytic stability of CoO/NF was tested by chronoamperometry at −0.3 V (vs. RHE) for 2 h over four cycles. The current density of CoO/NF did not decay after four cycles (Fig. 3c), and the conversion rate remained at 86.41% after four cycles (Fig. 3d), demonstrating excellent stability.
3.3 ECH mechanism
Further product analysis of constant-potential hydrogenation indicated that no tetrahydroquinoxaline was detected in the system after the addition of t-BuOH, with only a small amount of dihydroquinoxaline observed (Fig. 3f). These results confirm the central role of H* from the perspective of product distribution. t-BuOH competed with quinoxaline for H* capture, leading to a significant reduction in the concentration of active hydrogen species available for the hydrogenation reaction, thereby preventing quinoxaline from completing the deep hydrogenation process from the dihydroquinoxaline intermediate to the tetrahydroquinoxaline product. The ECH of quinoxaline depends highly on the supply of H* generated in the Volmer step, and the inhibitory effect of t-BuOH directly reveals the key mediating role of H* in the hydrogenation pathway, providing experimental evidence for the rationality of the “active hydrogen transfer” step in the reaction mechanism.35
3.3.2.1 Volmer reaction. This is the initial step of the alkaline HER and one of the rate-limiting steps of the reaction. On the catalyst surface, water molecules gain electrons and dissociate to form adsorbed hydrogen atoms (H*) and hydroxide ions (OH−). The reaction equation is as follows:
H2O + e− ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) H* + OH− H* + OH− | (6) |
This process was more difficult under alkaline conditions than under acidic conditions. The number of protons was limited in an alkaline environment, requiring the hydrogen–oxygen bond of water molecules to be broken first, a step that is relatively slow.
3.3.2.2 Heyrovsky reaction. Hydrogen desorption produced hydrogen gas. Hydrogen atoms (H*) in the adsorbed state reacted with water molecules in the electrolyte, combining with a proton (H+) and gaining an electron to form hydrogen gas molecules (H2) while also producing hydroxide ions (OH−).
H* + H2O + e− ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) H2 + OH− H2 + OH− | (7) |
This step involved the conversion of adsorbed hydrogen into hydrogen molecules.
3.3.2.3 Tafel reaction. Two hydrogen atoms adsorbed on the catalyst surface directly combine to produce H2, with the following reaction equation:
H* + H* ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) H2 H2 | (8) |
This reaction was more likely to occur at higher overpotentials and the main reaction pathway on some HER catalysts. The overall reaction equation for the electrochemical HER under alkaline conditions is as follows:
2H2O + 2e− ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) H2 + 2OH− H2 + 2OH− | (9) |
The role of an electrocatalyst is to reduce the reaction activation energy, thereby improving the faradaic efficiency and HER rate. Different catalysts promoted each reaction step to different degrees, which also accounted for their differences in HER performance under alkaline conditions. After the addition of quinoxaline, the second step of water electrolysis, the Heyrovsky reaction and Tafel reaction, competes with the quinoxaline hydrogenation reaction. Hydrogen protons can react with quinoxaline to form dihydroquinoxaline and further hydrogenate to form tetrahydroquinoxaline, or they can form hydrogen gas.
After the Volmer reaction occurs during water electrolysis, adsorbed active hydrogen atoms (H*) are generated. These active hydrogen atoms (H*) can undergo three types of reactions:
(i) Heyrovsky reaction
The adsorbed hydrogen atom (H*) reacts with water molecules in the electrolyte, combining with a proton (H+) and gaining an electron to form hydrogen gas molecules (H2) while also producing hydroxide ions (OH−).
(ii) Tafel reaction
Two hydrogen atoms (H*) adsorbed on the catalyst surface directly combine to form hydrogen gas molecules (H2).
(iii) Quinoxaline hydrogenation reaction
The active center of CoO/NF comprises Co2+ and the oxygen vacancy, which synergistically functions during quinoline hydrogenation. Co2+, leverages its unfilled d orbitals as a Lewis acid site, forms a Lewis acid–base pair with the lone pair electrons of the nitrogen atom in the quinoline, enables strong adsorption of the organic molecule.39–42 Simultaneously, the oxygen vacancy promotes the dissociation of interfacial water molecules under cathodic potential, formes a dominant structure characterized by two-coordinate hydrogen bonds (2-HB). These significantly reduces the water dissociation energy barrier, enables the continuous generation of highly reactive hydrogen atoms (H*) on the catalyst surface via the Volmer step. This process provides a hydrogen source for the efficient hydrogenation of quinoline.43,44 Step 1 represents the generation of H* on the catalyst surface via the Volmer step,45,46 while the cathode electrons are injected into the LUMO of quinoxaline (Fig. 4). Step 2 involves H* selectively attacking the highly electrophilic C3 position to form a semihydrogenated radical intermediate (QXL-I). The quinoxaline radical QXL-I remains adsorbed on the catalyst surface and continues to accept electrons, being reduced to a negatively charged intermediate. At this point, the newly generated H* in Step 3 rebinds with the intermediate, completing the addition of the second hydrogen atom at the adjacent unsaturated bond position of the pyridine ring and forming dihydroquinoxaline (QXL-II).36 In this stage, one of the two double bonds in the pyridine ring is fully saturated, while the molecule retains part of its unsaturated structure. The dihydroquinoxaline intermediate remains adsorbed, and in Step 4, it further accepts electrons and reacts with H*. The third hydrogen atom is added to the remaining unsaturated carbon–nitrogen bond of the pyrazine ring, forming the quinoxaline radical QXL-III intermediate. At this point, only one unsaturated bond remains in the pyrazine ring, and the molecular polarity is enhanced because of hydrogenation, gradually reducing the binding energy with the catalyst surface. In the final step, Step 5, the quinoxaline radical QXL-III intermediate accepts the final electron and undergoes a final addition reaction with the newly generated H*, completely saturating all the unsaturated bonds in the pyridine ring and ultimately forming tetrahydroquinoxaline. The product spontaneously desorbs because of the significantly reduced energy of binding with the catalyst surface, releasing the active site and completing the entire hydrogenation cycle.
4 Conclusions
CoO/NF with a nanocrystalline structure was used for the ECH of quinoxaline. CoO/NF overcomes the HER competition limitation through Co2+ site-specific adsorption and hydrogen transfer pathway reconstruction, enabling hydrogen storage in LOHCs at room temperature when water is used as the hydrogenation proton. At room temperature and atmospheric pressure, the rate of quinoxaline conversion and the selectivity rate for tetrahydroquinoxaline reached 99.84% and 98.73%, respectively. Additionally, after four cycles of stability testing, the rate of reaction completion remained at 86.41%, confirming the significant long-term stability of CoO/NF in terms of catalytic performance and structure. CoO/NF exhibited outstanding electrocatalytic performance in this system, with Co2+ providing critical substrate adsorption activation sites and oxygen vacancies for efficient water splitting to produce H* while regulating the reaction pathway and selectivity. Furthermore, the protons required for quinoxaline hydrogenation are entirely derived from water in the alkaline electrolyte, eliminating the need for external hydrogen. Therefore, the reversible electrochemical hydrogen storage system using CoO/NF as the electrode exhibits outstanding performance.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: electrocatalytic hydrogenation performance of nitrogen-containing heterocyclic organic molecules. See DOI: https://doi.org/10.1039/d5se01363a.Acknowledgements
This work was supported by the R&D Project of China Southern Power Grid Co., Ltd (YNKJXM20240023).References
- F. M. Bagwan, A. K. Kinage and S. N. Vasireddy, Reaction kinetics for catalytic hydrogenation of quinoline to decahydroquinoline as liquid organic hydrogen carrier, Int. J. Hydrogen Energy, 2024, 92, 102–112, DOI:10.1016/j.ijhydene.2024.10.247.
- R. Gunasekar, R. L. Goodyear, I. P. Silvestri and J. Xiao, Recent developments in enantio- and diastereoselective hydrogenation of N-heteroaromatic compounds, Org. Biomol. Chem., 2022, 20, 1794–1827, 10.1039/d1ob02331d.
- Z.-H. He, N. Li, K. Wang, W.-T. Wang and Z.-T. Liu, Selective hydrogenation of quinolines over a CoCu bimetallic catalyst at low temperature, Mol. Catal., 2019, 470, 120–126, DOI:10.1016/j.mcat.2019.04.005.
- R. Jiang, X. Zheng, C. Wang, W. Wei, T. Jin, Y. Zhao and Z. Zhang, Chemoselective hydrogenation of quinolines and imines using unsupported nanoporous ruthenium catalyst, Tetrahedron, 2024, 162, 134091, DOI:10.1016/j.tet.2024.134091.
- X. Chu, G. Zhou, M. Pang and H. Zhang, Manganese-catalysed transfer hydrogenation of quinolines under mild conditions, Eur. J. Org Chem., 2023, 26, e202300597, DOI:10.1002/ejoc.202300597.
- S. Guo, Y. Wu, C. Wang, Y. Gao, M. Li, B. Zhang and C. Liu, Electrocatalytic hydrogenation of quinolines with water over a fluorine-modified cobalt catalyst, Nat. Commun., 2022, 13, 5297, DOI:10.1038/s41467-022-32933-6.
- J. Huang, J. Li, Y. Cai and S. Zhao, Enhancing the cycle stability of milled Mg-Ni alloys: The role of Pd substitution on reversible electrochemical hydrogenation/dehydrogenation reactions, Electrochem. Commun., 2025, 170, 107860, DOI:10.1016/j.elecom.2024.107860.
- F. Z. Kashani and M. Mohsennia, Enhancing electrochemical hydrogen storage in nickel-based metal-organic frameworks (MOFs) through zinc and cobalt doping as bimetallic MOFs, Int. J. Hydrogen Energy, 2025, 101, 348–357, DOI:10.1016/j.ijhydene.2024.12.456.
- N. Shida, Y. Shimizu, A. Yonezawa, J. Harada, Y. Furutani, Y. Muto, R. Kurihara, J. N. Kondo, E. Sato, K. Mitsudo, S. Suga, S. Iguchi, K. Kamiya and M. Atobe, Electrocatalytic hydrogenation of pyridines and other nitrogen-containing Aromatic Compounds, J. Am. Chem. Soc., 2024, 146, 30212–30221, DOI:10.1021/jacs.4c09107.
- P. Zhang and L. Sun, Electrocatalytic hydrogenation and oxidation in aqueous conditions, Chin. J. Chem., 2020, 38, 996–1004, DOI:10.1002/cjoc.201900467.
- V. Vermaak, H. C. M. Vosloo and A. J. Swarts, Fast and efficient nickel(II)-catalysed transfer hydrogenation of ouinolines with ammonia borane, Adv. Synth. Catal., 2020, 362, 5788–5793, DOI:10.1002/adsc.202001147.
- M. Li, C. Liu, Y. Huang, S. Han and B. Zhang, Water-involving transfer hydrogenation and dehydrogenation of N-heterocycles over a bifunctional MoNi4 electrode, Chin. J. Catal., 2021, 42, 1983–1991, DOI:10.1016/S1872-2067(21)63834-2.
- S. Wang, S. Zhang, S. Liu, Z. Zhang, Y. Tan and K. Liang, Highly selective interconversion of quinoxaline and 1,2,3,4-tetrahydroquinoxaline over a spinel CuCo2O4 electrocatalyst supported by Ni foam, Chem. Phys. Lett., 2024, 856, 141638, DOI:10.1016/j.cplett.2024.141638.
- S. Wang, S. Zhang, Z. Zhang, X. Guo, Y. Tan, K. Liang and X. Wang, Efficient electrochemical hydrogenation and dehydrogenation of quinoxaline over a dendritic structure P-WO3/NF electrode, Mol. Catal., 2024, 554, 113842, DOI:10.1016/j.mcat.2024.113842.
- S. Wang, S. Zhang, Z. Zhang, Y. Tan, K. Liang, X. Guo and X. Kong, Reversible electrochemical hydrogen storage of quinoxaline utilizing Pd/NF dual-function electrocatalyst under mild conditions, Int. J. Hydrogen Energy, 2024, 49, 719–728, DOI:10.1016/j.ijhydene.2023.07.089.
- Z. Zhang, S. Zhang, S. Wang, Y. Tan, K. Liang, X. Guo and X. Zheng, Quinoline reversible electrochemical hydrogen storage on phosphide transition metal catalysts, Mol. Catal., 2025, 577, 114964, DOI:10.1016/j.mcat.2025.114964.
- D. Guo, L. Wen, T. Wang and X. Li, Electrodeposition synthesis of cobalt-molybdenum bimetallic phosphide on nickel foam for efficient water splitting, J. Colloid Interface Sci., 2024, 659, 707–717, DOI:10.1016/j.jcis.2023.09.173.
- Q. Ren, H. Jin, X. Xu, A. Liu, J. Li, J. Wang and S. Wang, Hydrogen evolution reaction catalyzed by nickel/nickel phosphide nanospheres synthesized through electrochemical methods, Electrochim. Acta, 2019, 298, 229–236, DOI:10.1016/j.electacta.2018.12.087.
- D. Ahmadkhaniha, D. Ascani, M. Fedel and C. Zanella, Electrodeposition and properties of Ni-Co-W-(Mo-Cu) high/medium entropy alloy coatings deposited from an aqueous bath, Intermetallics, 2025, 181, 108744, DOI:10.1016/j.intermet.2025.108744.
- L. Sun, Q. Gu, J. A. Yuwono, J. Zhou, B. Johannessen, L. Zhao, C. Zhang, G. Li, Z. Guo and S. Zhang, High-performance aprotic Li-CO2 battery enabled by the Ru heterophase catalyst, ACS Nano, 2025, 19(21), 20051–20062, DOI:10.1021/acsnano.5c03827.
- A. Niciejewska, G. Dercz, I. Matuła, A. Żak, W. Tylus, A. Laszczyńska and J. Winiarski, The use of DES green solvents to produce electrocatalytically active Ni-Mo alloys. The insight into the role of surface chemistry and mechanism of HER, Int. J. Hydrogen Energy, 2024, 96, 21–34, DOI:10.1016/j.ijhydene.2024.11.243.
- Y. Li, M. Wang, Y. Huang, H. Yang and T. Hang, An electrolyte for electrodeposition of hard gold based on choline chloride-urea ionic liquid, Electrochem. Commun., 2023, 148, 107454, DOI:10.1016/j.elecom.2023.107454.
- I. M. A. Omar, A. M. Al-Fakih, M. Aziz and K. M. Emran, Part II: Impact of ionic liquids as anticorrosives and additives on Ni-Co alloy electrodeposition: Experimental and DFT study, Arabian J. Chem., 2021, 14, 102909, DOI:10.1016/j.arabjc.2020.11.015.
- L. Anicai, I. Sin, O. Brincoveanu, S. Costovici, A. Cotarta, A. Cojocaru, M. Enachescu and T. Visan, Electrodeposition of lead selenide films from ionic liquids based on choline chloride, Appl. Surf. Sci., 2019, 475, 803–812, DOI:10.1016/j.apsusc.2018.12.191.
- Y. Xiao, X. Chen, T. Li, Y. Mao, C. Liu, Y. Chen and W. Wang, Mo-doped cobalt hydroxide nanosheets coupled with cobalt phosphide nanoarrays as bifunctional catalyst for efficient and high-stability overall water splitting, Int. J. Hydrogen Energy, 2022, 47, 9915–9924, DOI:10.1016/j.ijhydene.2022.01.071.
- Y. Guo, F. Liu, L. Feng, X. Wang, X. Zhang and J. Liang, Single Co atoms anchored on nitrogen-doped hierarchically ordered porous carbon for selective hydrogenation of quinolines and efficient oxygen reduction, Chem. Eng. J., 2022, 429, 132150, DOI:10.1016/j.cej.2021.132150.
- W.-Y. Chen, Y.-A. Yang and I. W. P. Chen, Nanosheet-engineered CoZMNS@NF heterostructure catalysts for seawater electrolysis toward sustainable hydrogen production, Chem. Eng. J., 2025, 520, 166045, DOI:10.1016/j.cej.2025.166045.
- S. Wu, X. Huang, H. Zhang, Z. Wei and M. Wang, Efficient electrochemical hydrogenation of nitroaromatics into arylamines on a CuCo2O4 spinel cathode in an alkaline electrolyte, ACS Catal., 2022, 12, 58–65, DOI:10.1021/acscatal.1c03763.
- H. Yu, J. Li, N. Yang, D. Zhao, B. Yang, Y. Hou and G. Xie, Ultrafast Joule heating method for synthesizing CoNi nanoalloy catalysts for electrocatalytic hydrogen evolution, J. Alloys Compd., 2025, 1019, 179101, DOI:10.1016/j.jallcom.2025.179101.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni, Appl. Surf. Sci., 2011, 257, 2717–2730, DOI:10.1016/j.apsusc.2010.10.051.
- Z. Zhang, X. Guo, Y. Li, R. Liu, Y. Tan, K. Liang and S. Zhang, Bifunctional electrodes enable efficient electrochemical cycling of quinoline, Chem. Phys. Lett., 2025, 877, 142196, DOI:10.1016/j.cplett.2025.142196.
- X. Zhao, K. Gao, S. Xue, W. Ran and R. Liu, Synergistic effects of oxygen vacancies and Pd single atoms on Pd@TiO2−x for efficient HER catalysis, Chin. Chem. Lett., 2025, 36, 110309, DOI:10.1016/j.cclet.2024.110309.
- X. Li, J. Yu, H. Zhang, G. Liu, S. Luo, J. Guo, Z. Li and Y. Zhang, Structural evolution of amorphous CoMo alloy in alkaline based hydrogen evolution reaction, Appl. Surf. Sci., 2025, 699, 163161, DOI:10.1016/j.apsusc.2025.163161.
- Z. Zhang, S. Zhang, S. Wang, X. Guo, Z. Wang, Y. Tan and K. Liang, Highly efficient electrochemical hydrogenation and dehydrogenation of quinoline catalyzed by a bifunctional RuNi electrode, Int. J. Hydrogen Energy, 2025, 114, 81–88, DOI:10.1016/j.ijhydene.2025.03.054.
- Y. Li, L. Li, S. Xu, K. Cui, T. Wang, Z. Jiang and J. Li, Hydrogen spillover mechanism at the metal-metal interface in electrocatalytic hydrogenation, Angew. Chem., Int. Ed., 2024, 63, e202407810, DOI:10.1002/anie.202407810.
- M. Rosales, F. Arrieta, P. Baricelli, A. Colina and R. Izquierdo, A detailed DFT theoretical investigation of the mechanism of quinoline hydrogenation catalyzed by a (1,5-cyclooctadiene)rhodium(I) complex, Catal. Today, 2025, 447, 115160, DOI:10.1016/j.cattod.2024.115160.
- H. Luo, S. Wei, P. Xing, Y. Wang and L. Dai, Ni-CoS2 nanoparticles loaded on 3D RGO for efficient electrochemical hydrogen and oxygen evolution reaction, J. Electroanal. Chem., 2024, 974, 118713, DOI:10.1016/j.jelechem.2024.118713.
- X. Luo, L. Jiao, D. Chao, F. Li, R. Wang, S. Zhang, Q. Ma, H. Li, L. Zhang and C. Zhang, Synergistic effects of electrolyte additives in a dual-salt system for high-performance four electron aqueous zinc-iodine batteries across a wide temperature range, Angew. Chem., Int. Ed., 2025, 64, e202514375, DOI:10.1002/anie.202514375.
- J. Nie, Z. Zhu, Y. Liao, X. Xiao, F. Mauriello and Z. Zhang, Highly efficient and selective hydrogenation of quinolines at room temperature over Ru@NC-500 catalyst, Mol. Catal., 2022, 526, 112381, DOI:10.1016/j.mcat.2022.112381.
- K. Naveen, T. Mahvelati-Shamsabadi, P. Sharma, S.-h. Lee, S. H. Hur, W. M. Choi, T. J. Shin and J. S. Chung, MOF-derived Co/Zn single-atom catalysts for reversible hydrogenation and dehydrogenation of quinoline hydrogen carrier, Appl. Catal., B, 2023, 328, 122482, DOI:10.1016/j.apcatb.2023.122482.
- F. Tang, G. Zhang, L. Wang, J. Huang and Y.-N. Liu, Unsymmetrically N, S-coordinated single-atom cobalt with electron redistribution for catalytic hydrogenation of quinolines, J. Catal., 2022, 414, 101–108, DOI:10.1016/j.jcat.2022.08.033.
- Y. Liu, S. Yang, X. You, L. Qin, Y. Qin, W. Zhang and W. Liang, Electrocatalytic hydrogenation of nitrogenous heterocyclics using Co-based nanoparticles-embedded N-doped carbon derived from ZIF-67, J. Alloys Compd., 2022, 921, 165940, DOI:10.1016/j.jallcom.2022.165940.
- H. Du, T. Wang, M. Li, Z. Yin, R. Lv, M. Zhang, X. Wu, Y. Tang, H. Li and G. Fu, Identifying highly active and selective cobalt X-ides for electrocatalytic hydrogenation of quinoline, Adv. Mater., 2024, 36, 2411090, DOI:10.1002/adma.202411090.
- W. Zhang, Y. Liu, X. Luo, R. Wang, K. Zhou, L. Yuan, F. Li, H. Li, L. Zhang and C. Zhang, Multi-solvent synergy strategy unlocks anti-corrosion and high reversibility of zinc anodes: paving the way for robust and temperature-resilient zinc-iodine batteries, Adv. Funct. Mater., 2025, e12633, DOI:10.1002/adfm.202512633.
- W. Zhang, R. Gao, J. Chen, J. Wang, J. Zheng, L. Huang and X. Liu, Water-induced surface reconstruction of Co3O4 on the (111) plane for high-efficiency Li-O2 batteries in a hybrid electrolyte, ACS Appl. Mater. Interfaces, 2022, 14, 28965–28976, DOI:10.1021/acsami.2c06990.
- M. Qin, J. Chen, M. Qi, H. Wang, S. Mao, L. Xi and Y. Wang, Constructing heteronuclear bridging atoms toward bifunctional electrocatalysis, ACS Catal., 2024, 14, 8414–8426, DOI:10.1021/acscatal.4c01705.
| This journal is © The Royal Society of Chemistry 2026 |



