Highly stable Ni(II)/Zn(II)-CPs as multiresponsive luminescent sensors for detection of IO4−, 2,6-dichloro-4-nitrophenol and folic acid†
Fang-Hua
Zhao
 *,
Zi-Hao
Zhao
,
Rui
Feng
,
Yu-Shuo
Li
,
Shu-Qi
Li
,
Zhi-Hong
Jing
*,
Zi-Hao
Zhao
,
Rui
Feng
,
Yu-Shuo
Li
,
Shu-Qi
Li
,
Zhi-Hong
Jing
 and
Zhong-Lin
Li
*
and
Zhong-Lin
Li
*
Key Laboratory of Life-Organic Analysis of Shandong Province, School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu 273165, China. E-mail: zfh101@163.com; lizhonglin788@163.com
First published on 25th June 2024
Abstract
Coordination polymer (CP) materials have an important application as sensors. In this work, two new Ni(II)/Zn(II)-CPs of sebacic acid (H2seb) and 1,6-bis(benzimidazole)hexane (bbimh), {[Ni3(seb)3(bbimh)4]·7H2O}n (1) and {[Zn(seb)(bbimh)]·H2O}n (2), were constructed as luminescent sensors. Ni(II)-CP 1 features a three-dimensional (3D) (4,5)-connected framework including 1D channels occupied by water molecules. Zn(II)-CP 2 shows a 3D supramolecular network with 6-connected pcu topology. Both CPs have good stability in aqueous solution as well as in EtOH. Luminescence sensing studies reveal that 1 and 2 display high luminescence quenching efficiency toward IO4−, 2,6-dichloro-4-nitrophenol (2,6-DCNP) and folic acid (FA). Both CPs also display excellent sensitivity with limits of detection toward IO4−, 2,6-DCNP and FA as low as 1.23 μM, 13.26 nM and 13.04 μM for 1 and 1.26 μM, 30.61 nM and 15.70 μM for 2, respectively. Additionally, the two CPs were also demonstrated to be ultrasensitive and selective sensors for the detection of IO4− in swimming pool water, 2,6-DCNP in farmland sewage and FA in human serum. The experimental results suggest that the synthesized Ni(II)/Zn(II)-CPs have potential application as luminescence sensing materials in environmental pollution and human health.
1. Introduction
As an important oxidising agent, periodate (IO4−) can be converted to iodine-containing compounds in the lower valence state and is widely used in organic synthesis and catalysis of biomolecules as well as in disinfection processes.1–31 In the human body, IO4− is found mainly in the thyroid tissue, some of which is taken up by thyroid cells and is involved in the synthesis of thyroid hormones. However, it has been reported that excess IO4− ions in the body may cause hyperthyroidism by blocking the release of thyroid hormones.4–6 2,6-Dichloro-4-nitrophenol (2,6-DCNP) is a common chemical that has a wide range of applications in pesticides.7 As a pesticide ingredient, it is effective in controlling the growth of pests and pathogens and protecting healthy crops. The main reason for using 2,6-DCNP in pesticides is that it has good bactericidal and insecticidal effects. It destroys the cell membranes and cell walls of pests and pathogens, preventing their growth and reproduction.8–10 Folic acid (FA, also known as vitamin B9) is a water-soluble vitamin that plays an important role in the body.11 It maintains normal cell growth and division, supports the development of the nervous system in the fetus, promotes the production of red blood cells and helps maintain normal immune cell activity.12–14 However, too much FA can be harmful to the body in a number of ways. Excess folic acid masks vitamin B12 deficiency, masks symptoms of anemia, and increases the risk of colon and prostate cancers.15,16 Based on the damage caused by excess amounts of the above substances to the human body, the development of a convenient and fast testing method is urgently needed.To date, a variety of materials have been designed and synthesized for use as probes to detect IO4−, 2,6-DCNP and FA. These materials include organic dyes,17 metal nanoparticles,18 quantum dots,19 and coordination polymers.20–23 Coordination polymers (CPs) are polymers formed by ligands and central metal ions through coordination bonds. Compared with other probe materials, CPs have unique advantages such as reversibility, tunability, and versatility, making them valuable for important applications in analysis and detection.24–26 These unique advantages enable CPs to possess properties such as luminescence sensing,27–30 electrocatalysis,31 drug delivery,32 gas adsorption,33 photocatalysis,34,35 magnetism36 and antibacterial properties.37 CPs as fluorescent sensors have been widely used for the detection of analytes such as cations,38 anions,39 volatile organic compounds (VOCs)40 and bioanalytes,41–43 while multiresponsive luminescent sensors for the detection of IO4−, 2,6-DCNP and FA are relatively rare. Therefore, the development of new CP-based luminescent sensors for detection of IO4−, 2,6-DCNP and FA is highly necessary.
In the synthesis of CPs, the selection of suitable ligands plays an important role in their structures and properties. To obtain CPs with excellent luminescence, the ligands containing abundant π-electrons are always selected because the π-conjugated systems can absorb energy and emit luminescence.44–46 In addition, the introduction of –CH2– in the ligand can provide flexible conformational freedom, which in turn affects the stereoconformation and properties of the CPs. 1,6-Bis(benzimidazole)hexane (bbimh) as a N-donor ligand with two benzimidazole rings and a –(CH2)6– spacer is an excellent ligand to construct new CPs with various structures and luminescence. Furthermore, the mixed ligand strategy is an effective approach for the construction of new CPs. Aliphatic carboxylic acids are available in a wide range of lengths and substituent choices, and the properties and structures of CPs can be modulated by adjusting different aliphatic chain lengths and types of carboxylic acid groups. In addition, aliphatic carboxylic acids have good coordination ability and can build stable coordination bonds with metal ions. Sebacic acid (H2seb) is a good aliphatic dicarboxylic acid,47,48 and upon careful inspection, no CPs based on bbimh and H2seb have been reported.
In this contribution, two new stable Ni(II)/Zn(II)-CPs based on the mixed ligand strategy were obtained by a hydrothermal method. Both CPs show good luminescence stability, which encourages us to explore their application as luminescent sensors. Luminescence sensing experimental results indicate that both CPs are multifunctional luminescent sensors for the detection of IO4−, 2,6-DCNP and FA with high selectivity and sensitivity. The two CPs were also applied to detect IO4− in swimming pool water, 2,6-DCNP in farmland sewage and FA in human serum, respectively. They both show the fluorescence quenching (turn off) behavior toward IO4−, 2,6-DCNP and FA based on a competition absorption mechanism. Furthermore, the fabricated films of two CPs were also prepared, which can be used for visual inspection by the naked eye.
2. Experimental section
2.1 Materials and instruments
Materials and instruments are shown in the ESI.†2.2 Synthesis
Synthesis of {[Ni3(seb)3(bbimh)4]·7H2O}n (1). Ni(NO3)2·6H2O (0.50 mmol, 145 mg), H2seb (0.50 mmol, 101 mg), and bbimh (0.50 mmol, 160 mg) were added to 10 mL of distilled water and the mixture was stirred at room temperature for 0.5 h. The pH of the mixture was then adjusted to 6.0 using 0.5 M sodium hydroxide solution. Next, the mixture was placed in a Teflon lined stainless steel vessel. It was heated for 3 days at 160 °C. After cooling to room temperature, light green crystals were obtained (yield: 58%, based on Ni salt). Elemental analysis calcd: C, 60.70%; H, 6.95%; N, 10.30%. Found: C, 60.92%; H, 7.19%; N, 10.12%. IR (KBr, cm−1): 3395 w, 3117 m, 2929 s, 2853 m, 1700 w, 1614 w, 1545 s, 1509 m, 1463 s, 1399 s, 1335 w, 1296 m, 1247 m, 1195 m, 1161 w, 1095 w, 1010 w, 927 w, 811 w, 742 m, 635 w.Synthesis of {[Zn(seb)(bbimh)]·H2O}n (2). CP 2 was synthesized by the same synthesis method as that used for 1, except that Ni(NO3)2·6H2O was replaced with Zn(NO3)2·6H2O (0.50 mmol, 154 mg). After cooling to room temperature, colorless crystals were obtained (yield: 65%, based on Zn salt). Elemental analysis calcd: C, 59.85%; H, 6.70%; N, 9.31%. Found: C, 60.07%; H, 6.88%; N, 9.49%. IR (KBr, cm−1): 3390 w, 3099 m, 2925 s, 2851 m, 1706 w, 1614 m, 1534 s, 1465 m, 1398 m, 1336 w, 1299 w, 1268 w, 1244 w, 1199 m, 1165 w, 1121 m, 1050 m, 1000 m, 974 w, 925 w, 756 s, 742 m, 655 w, 616 w.
2.3 Luminescence sensing measurements
3 mg of ground samples of 1 and 2 were added to 3 mL of 1 mM aqueous solutions of 11 anions (CO32−, Ac−, I−, Br−, F−, Cl−, C2O42−, PO43−, ClO3−, SO42− and IO4−) or 20 μM ethyl alcohol solutions of 10 common agricultural chemicals (2,4,6-trichlorophenol (2,4,6-TCP), 3,4,5-trifluorophenol (3,4,5-TFP), 2,6-dichloro-4-nitrophenol (2,6-DCNP), chlorobenzene (PCB), imidazole (MZ), methyltin mercaptide (MTM), glycine methyl ester hydrochloride (GABA), permethrin (PREM), 4-amino-2,6-dichlorophenol (DCAP), and N-(phosphonomethyl)glycine (GLYP)), or 0.5 mM aqueous solutions of 9 biological analytes (folic acid (FA), glucose (Glc), uric acid (UA), creatinine (SCr), creatine (Cre), vitamin C (Vc), L-alpha-glutamyl-L-glutamate (Glu–Glu), dipicolinic acid (DPA) and adenine (A)), and sonicated for 0.5 h to form suspensions, and then the fluorescence emission spectra of the suspensions were tested at room temperature with a PMT voltage of 500 V and a slit width of 5 nm.2.4 Analysis in real samples
The swimming pool and farmland sewage water samples were filtered through a 0.22 μm filter membrane three times. Then the swimming pool and farmland sewage water samples were used in the sensing experiments. The normal human blood samples were obtained from the school hospital of Qufu Normal University. The obtained blood samples were centrifuged at 8000 rpm for 20 minutes. The serums were obtained after centrifugation and diluted one hundred times, and the diluted samples were added with different concentrations of folic acid for the sensing experiments.3. Results and discussion
3.1 Crystal structure of {[Ni3(seb)3(bbimh)4]·7H2O}n (1)
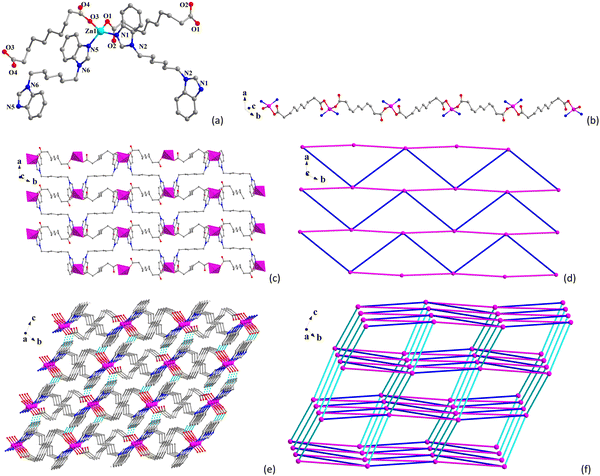
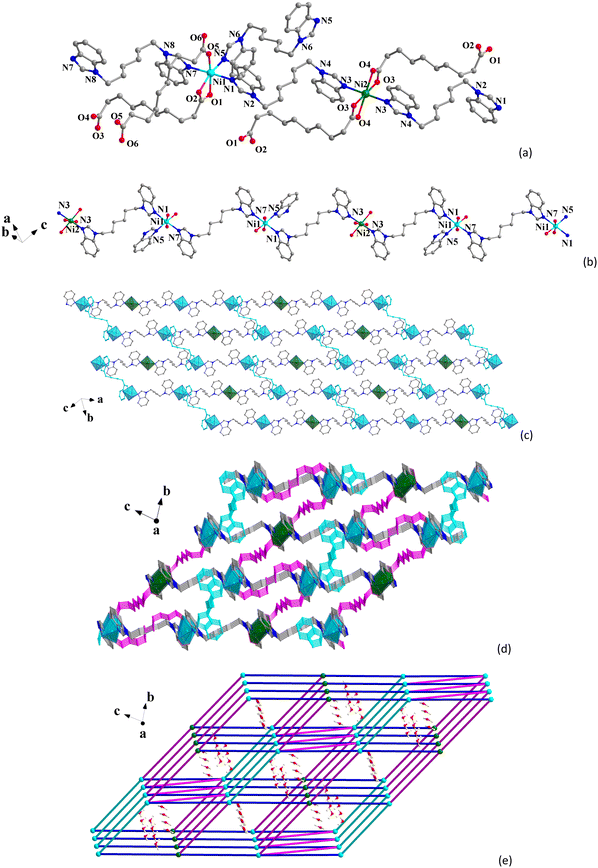
Single-crystal X-ray diffraction results indicate that CP 1 crystallizes in the monoclinic P![[1 with combining overline]](https://www.rsc.org/images/entities/char_0031_0305.gif) space group (Table S1, ESI†). The asymmetric unit contains one and a half Ni(II) ions, one and a half seb2−, two bbimh molecules, and three and a half free water molecules. As shown in Fig. 1a, the octahedral Ni1 center is surrounded by three O atoms (O1, O2 and O5) from two different seb2− and three N atoms (N1, N5 and N7) from three bbimh ligands. The octahedral Ni2 center is coordinated by four O atoms (O3 and O3#1, O4 and O4#1) from two seb2− and two N atoms (N3 and N3#1) from two bbimh ligands. The Ni–O bond lengths range from 2.017(2) to 2.157(2) Å, and the Ni–N bond lengths range from 2.065(2) to 2.152(2) Å (Table S2, ESI†), which are similar to other reported Ni(II)-CPs.49–51 The seb2− ligands adopt two coordinated modes with one seb2− linking with one Ni1 ion and one Ni2 ion in the μ2-κ1:κ1:κ1:κ1 chelating mode (Scheme S1I, ESI†) and the other seb2− linking with two Ni1 ions in the μ2-κ1:κ0:κ1:κ0 bridging mode (Scheme S1II, ESI†). The Ni1⋯Ni2 and Ni1⋯Ni1 distances through the seb2− ligands are 12.2040(7) and 12.3381(9) Å, respectively. The bbimh ligands all display the μ2-trans conformation (Scheme S1III, ESI†) connecting two Ni(II) ions with the Ni⋯Ni distances being 11.7917(8), 13.5141(7) and 13.9245(9) Å.
space group (Table S1, ESI†). The asymmetric unit contains one and a half Ni(II) ions, one and a half seb2−, two bbimh molecules, and three and a half free water molecules. As shown in Fig. 1a, the octahedral Ni1 center is surrounded by three O atoms (O1, O2 and O5) from two different seb2− and three N atoms (N1, N5 and N7) from three bbimh ligands. The octahedral Ni2 center is coordinated by four O atoms (O3 and O3#1, O4 and O4#1) from two seb2− and two N atoms (N3 and N3#1) from two bbimh ligands. The Ni–O bond lengths range from 2.017(2) to 2.157(2) Å, and the Ni–N bond lengths range from 2.065(2) to 2.152(2) Å (Table S2, ESI†), which are similar to other reported Ni(II)-CPs.49–51 The seb2− ligands adopt two coordinated modes with one seb2− linking with one Ni1 ion and one Ni2 ion in the μ2-κ1:κ1:κ1:κ1 chelating mode (Scheme S1I, ESI†) and the other seb2− linking with two Ni1 ions in the μ2-κ1:κ0:κ1:κ0 bridging mode (Scheme S1II, ESI†). The Ni1⋯Ni2 and Ni1⋯Ni1 distances through the seb2− ligands are 12.2040(7) and 12.3381(9) Å, respectively. The bbimh ligands all display the μ2-trans conformation (Scheme S1III, ESI†) connecting two Ni(II) ions with the Ni⋯Ni distances being 11.7917(8), 13.5141(7) and 13.9245(9) Å.
 | ||
| Fig. 1 (a) Coordination environment of Ni(II) ions in 1. (b) 1D chain of 1. (c) 2D layer of 1. (d) 3D structure of 1. (e) The binodal (4,5)-connected framework of 1. | ||
As shown in Fig. 1b, Ni(II) ions are connected through bbimh ligands to form 1D chains, and the 1D chains further extend to form the 2D layer via bbimh ligands (Fig. 1c), and the adjacent 2D layers are further connected by seb2− ligands to generate the 3D structure (Fig. 1d). As analyzed from the topological review, each Ni1 ion is connected to three neighboring Ni1 and two Ni2 ions through two seb2− and three bbimh ligands, which can be viewed as 5-connected nodes. Each Ni2 ion is connected to four neighboring Ni1 ions via two seb2− and two bbimh ligands, which can be viewed as a 4-connected node. Thus, the 3D framework of 1 can be simplified to (4,5)-connected binodal net with a Schläfli symbol of (44·62)(44·66)2 (Fig. 1e). Viewing from the a-axis reveals that CP 1 contains 1D channels occupied by lattice water molecules. The pore volume of 1 calculated by PLATON is 137.8 Å3, which corresponds to about 5.1% of the unit cell (2680.2 Å3).
3.2 Crystal structure of {[Zn(seb)(bbimh)]·H2O}n (2)
Single-crystal X-ray analysis shows that CP 2 crystallizes in the monoclinic P![[1 with combining overline]](https://www.rsc.org/images/entities/char_0031_0305.gif) space group. The asymmetric unit consists of a Zn(II), two half seb2−, two half bbimh and a free water molecule. The Zn(II) center displays a tetrahedral ZnN2O2 geometry (Fig. 2a), which is coordinated by two O atoms (O1 and O3) from two seb2− and two N atoms (N1 and N6) from two bbimh ligands. The bond lengths of Zn–O are 1.9551(19) Å and 1.973(2) Å, and the bond lengths of Zn–N are 2.006(2) Å and 2.007(2) Å, which are similar to other reported Zn(II)-CPs.52–54 The seb2− ligands all adopt the μ2-κ1:κ0:κ1:κ0 mode connecting with two Zn(II) ions (Scheme S1II, ESI†), and the Zn⋯Zn distances through two different seb2− are 10.9853(9) and 11.0846(9) Å. Two different bbimh ligands both connect to two Zn(II) ions with the μ2-trans conformation (Scheme S1III, ESI†) with the Zn⋯Zn distances being 13.5880(11) and 13.8140(9) Å.
space group. The asymmetric unit consists of a Zn(II), two half seb2−, two half bbimh and a free water molecule. The Zn(II) center displays a tetrahedral ZnN2O2 geometry (Fig. 2a), which is coordinated by two O atoms (O1 and O3) from two seb2− and two N atoms (N1 and N6) from two bbimh ligands. The bond lengths of Zn–O are 1.9551(19) Å and 1.973(2) Å, and the bond lengths of Zn–N are 2.006(2) Å and 2.007(2) Å, which are similar to other reported Zn(II)-CPs.52–54 The seb2− ligands all adopt the μ2-κ1:κ0:κ1:κ0 mode connecting with two Zn(II) ions (Scheme S1II, ESI†), and the Zn⋯Zn distances through two different seb2− are 10.9853(9) and 11.0846(9) Å. Two different bbimh ligands both connect to two Zn(II) ions with the μ2-trans conformation (Scheme S1III, ESI†) with the Zn⋯Zn distances being 13.5880(11) and 13.8140(9) Å.
As shown in Fig. 2b, the Zn(II) centers are connected by seb2− to form 1D chain, and the 1D chain is further connected through bbimh ligands to form 2D layer (Fig. 2c). The 2D network is an irregular 4-connected sql net (Fig. 2d). Finally, the 2D layers are extended into a 3D supramolecular structure via C–H⋯O hydrogen bonds (Table S3, ESI,† and Fig. 2e). From topological view, each Zn(II) ion is connected to six neighboring Zn(II) ions through two seb2−, two bbimh ligands, and two hydrogen bonds. Therefore, the Zn(II) ion can be viewed as a 6-connected node, and the 3D supramolecular structure of 2 can be simplified to a 6-connected pcu net with a Schläfli symbol of (412·63) (Fig. 2f).
3.3 Structural comparison
As described above, CPs 1 and 2 demonstrate two distinct structures for their different metal centers though they are constructed by two identical ligands. There are some possible reasons for their differences. First, different metal ions own different electronic structures and form different numbers of coordination bonds with the ligands in the coordination process. Ni(II) ions in 1 all have 6-coordinated octahedral geometry, while Zn(II) ions in 2 all have 4-coordinated tetrahedral geometry. Second, seb2− ligands in two structures display different coordination modes. From the structural descriptions of 1 and 2, it can be found that there are two coordination modes of seb2− in 1, one is μ2-κ1:κ1:κ1:κ1, and the other is μ2-κ1:κ0:κ1:κ0. Whereas in 2, there is only one coordination mode of μ2-κ1:κ0:κ1:κ0. Finally, the ratio of reactants also plays an important role. Different ratios of ligands and metal centers have different selectivity in the coordination reaction, leading to the formation of coordination polymers with different structures. For the reported CPs containing bbimh ligands, most of them are constructed by bbimh mixed with aromatic carboxylic acids. Recently, Cui et al. reported Ni(II)/Zn(II)/Cd(II) CPs of the bbimh ligand, which showed three 2D structures.55,56 In the present work, the flexible H2seb was used as the mixed ligand and resulted in two new structures. 1 has a 3D structure with a (4,5)-connected topology. 2 has a 2D structure which is further connected by hydrogen bonds to form a 3D supramolecular structure.3.4 Purity, thermal stability and N2 sorption property
The powder X-ray diffraction (PXRD) results (Fig. S1, ESI†) show that the measured peak positions are consistent with the simulated ones, which indicates that the synthesized samples of 1 and 2 are of high purity. TGA was carried out to study the thermal stability of 1 and 2 (Fig. S2, ESI†), the weight loss of 4.82% for 1 from 30 to 170 °C can be attributed to the loss of water molecules (calcd: 5.13%). Between 170 and 278 °C, 1 remains stable with almost no weight loss. After 278 °C, the framework of 1 begins to collapse with a dramatic decrease. The weight loss of 3.49% for 2 between 30 and 117 °C can be due to the loss of free water molecules (calcd: 3.73%). The curve of 2 shows that it can remain stable at 300 °C, after which the structure begins to collapse. The results indicate that both 1 and 2 have good thermal stability. In addition, the N2 adsorption–desorption isotherms of 1 and 2 were tested at 77 K (Fig. S3, ESI†). The nitrogen uptakes of 1 and 2 reach the maximum values of 20.45 and 16.98 cm3 g−1 at 1 atm. The BET specific surface areas of 1 and 2 are 16.53 and 31.49 m2 g−1, respectively.3.5 IR and UV-vis absorption spectra
From the infrared spectra of 1 and 2 (Fig. S4, ESI†), the characteristic peaks of the hydroxyl group (–OH) for water molecules at 3395 cm−1 in 1 and 3390 cm−1 in 2 can be observed. The ν(Ar–H) stretching vibrations of the aromatic ring in the bbimh ligand for 1 and 2 are observed at 3117 and 3110 cm−1. The peaks at 2929 and 2853 cm−1 for 1 and those at 2925 and 2851 cm−1 for 2 should be due to the ν(C–H) vibrations of –CH2– group in bbimh and H2seb ligands. The characteristic bands of asymmetric and symmetric stretching of the carboxyl group are located at 1614 and 1399 cm−1 for 1 and 1614 cm−1 and 1398 cm−1 for 2. The ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) vibrations of the benzene ring can be found at 1545 to 1463 cm−1 for 1 and 1534 to 1465 cm−1 for 2, respectively. In addition, the δ(CH2) vibration is produced at 1296 cm−1 and 1299 cm−1 for 1 and 2, respectively. Finally, the peaks from 1161 cm−1 to 927 cm−1 in 1 and from 1165 to 925 in 2 can be attributed to the ν(C–C) vibration in seb2−. The solid-state UV-visible absorption spectra of 1, 2 and bbimh ligand were tested (Fig. S5a, ESI†). The broad band from 210 to 330 nm for the bbimh ligand can be ascribed to π → π* transition of the benzimidazole ring. The bands located at 288 nm for 1 and 292 nm for 2 are similar to the absorption band of the bbimh ligand, which can be attributed to the π → π* transition of bbimh ligands in their structures. A broad band observed at 410 nm in 1 may be due to low-energy electronic transition caused by charge transfer or local coordination between the ligand and Ni(II) cation.57 In addition, the broad band at 680 nm for 1 should be attributed the d → d transition of the Ni(II) cation. According to Tauc's relation of (Ahν)2 = B(hν − Eg),58 the band gaps are obtained as 4.23 and 3.78 eV for 1 and 2, respectively (Fig. S5b, ESI†).
C) vibrations of the benzene ring can be found at 1545 to 1463 cm−1 for 1 and 1534 to 1465 cm−1 for 2, respectively. In addition, the δ(CH2) vibration is produced at 1296 cm−1 and 1299 cm−1 for 1 and 2, respectively. Finally, the peaks from 1161 cm−1 to 927 cm−1 in 1 and from 1165 to 925 in 2 can be attributed to the ν(C–C) vibration in seb2−. The solid-state UV-visible absorption spectra of 1, 2 and bbimh ligand were tested (Fig. S5a, ESI†). The broad band from 210 to 330 nm for the bbimh ligand can be ascribed to π → π* transition of the benzimidazole ring. The bands located at 288 nm for 1 and 292 nm for 2 are similar to the absorption band of the bbimh ligand, which can be attributed to the π → π* transition of bbimh ligands in their structures. A broad band observed at 410 nm in 1 may be due to low-energy electronic transition caused by charge transfer or local coordination between the ligand and Ni(II) cation.57 In addition, the broad band at 680 nm for 1 should be attributed the d → d transition of the Ni(II) cation. According to Tauc's relation of (Ahν)2 = B(hν − Eg),58 the band gaps are obtained as 4.23 and 3.78 eV for 1 and 2, respectively (Fig. S5b, ESI†).
3.6 Luminescence properties
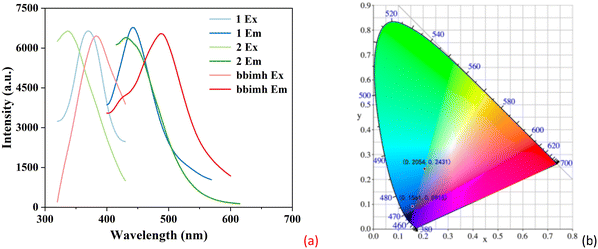
To study the luminescence properties of the two CPs, the luminescence spectra of the two CPs were measured. As shown in Fig. 3a, CPs 1 and 2 show the maximum emission peaks at 443 nm (λex = 370 nm) and 432 nm (λex = 338 nm), respectively, which are similar to the free bbimh ligand, suggesting that the luminescence of two CPs should be assigned to the π* → π and π*→ n transitions of the bbimh ligand in their structures.59 Furthermore, the CIE coordinates of 1 and 2 (Fig. 3b) are (0.2054, 0.2431) and (0.1561, 0.0915), respectively. In fluorescence sensing assays, stability is particularly important for subsequent sensing experiments. Therefore, the fluorescence stabilities of 1 and 2 with time in water and EtOH were measured (Fig. S6, ESI†). The fluorescence intensities of 1 and 2 in water and EtOH show almost no change within 24 hours. Furthermore, the fluorescence intensities of 1 and 2 were tested at different pH values. As shown in Fig. S6e and f (ESI†), there is no obvious change for the fluorescence intensities of 1 and 2 when the pH range from 2 to 12. In addition, the PXRD of 1 and 2 after immersed in water and EtOH for 24 hours, and in different pH values were performed (Fig. S7, ESI†). The experimental PXRD patterns are almost identical to those of the simulated ones. These results suggest that both 1 and 2 have excellent stabilities in water, EtOH and different pH values.3.7 Detection of inorganic anions
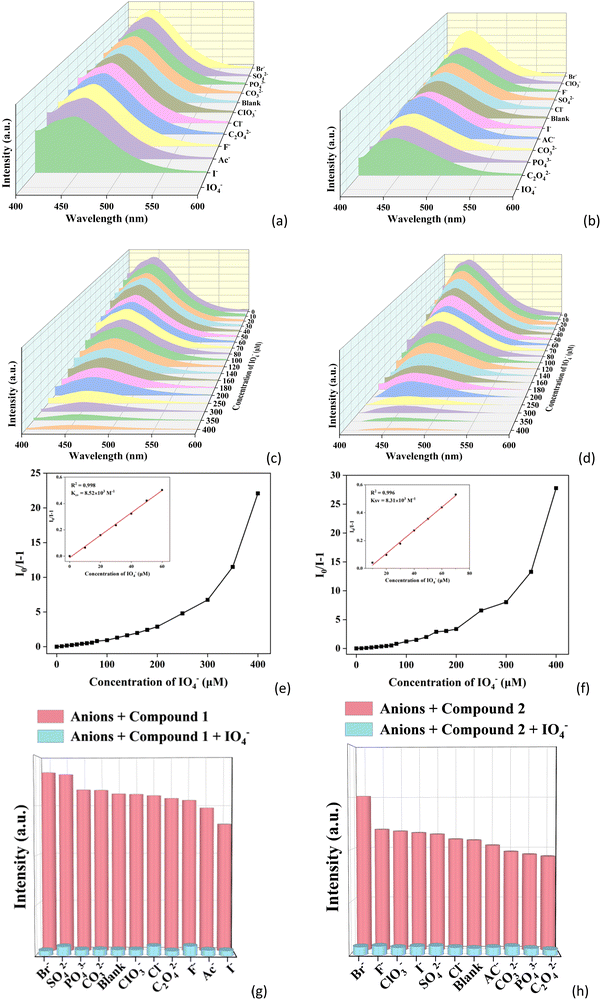
Inspired by the excellent luminescence properties and stability of 1 and 2, the luminescence sensing toward inorganic anions in water was investigated. Thus, the suspensions of 1 and 2 were introduced into various aqueous solutions of NaxA (A = CO32−, Ac−, I−, Br−, F−, Cl−, C2O42−, PO43−, ClO3−, SO42− and IO4−) with a concentration of 1 mM. The luminescence spectra of 1 and 2 in the presence of different inorganic anions were recorded. As illustrated in Fig. 4a and b, the most significant quenching effect is observed in the presence of IO4− ions, while other inorganic anions have negligible effect on the fluorescence intensity of 1 and 2 (Fig. S8a and b, ESI†), which can also be distinguished with the naked eye (Fig. S8c and d, ESI†). This suggests that 1 and 2 can serve as luminescent sensors to detect IO4− in water.Fig. 4c and d display the changes of fluorescence intensity for 1 and 2 in the presence of different concentrations of IO4− from 0 to 400 μM. It can be seen that the fluorescence intensity of 1 and 2 gradually decreases with the increase of IO4− concentration. Meanwhile, a linear relationship between the value of I0/I and the IO4− concentration in a lower concentration range was achieved (Fig. 4e and f). According to the Stern–Volmer equation,60I0/I − 1 = Ksv[M] (where I0 is the initial fluorescence intensity, I is the fluorescence intensity after the addition of IO4−, KSV is the quenching constant and [M] is the concentration of IO4−). The corresponding Ksv values were calculated to be 8.52 × 103 and 8.31 × 103 M−1 for CPs 1 and 2, respectively. Using the equation LOD = 3σ/Ksv, the limit of detection (LOD) values for CPs 1 and 2 toward IO4− were determined to be 1.23 and 1.26 μM, respectively, indicating that CPs 1 and 2 can be applied for efficient detection of IO4−.
In order to determine whether there is any interference from other anions during the identification of IO4− in the actual measurements, anti-interference experiments were carried out. As shown in Fig. 4g and h, the experimental results still show obvious quenching behavior of 1 and 2 when other anions and IO4− coexisted (Fig. S8e and f, ESI†). This implies that CPs 1 and 2 have high selectivity and excellent anti-interference ability for the detection of IO4−. In previous reported works, some CP-based luminescent sensors have been applied to detect inorganic anions, but there are only two CPs for detecting IO4− (Table S4, ESI†).61,62 CPs 1 and 2 are two new luminescent sensors of IO4−.
To investigate the feasibility of 1 and 2 as luminescent sensors of IO4− in real samples, they were applied to detect IO4− in swimming pool water. A similar approach was used as the above except that the deionized water was used instead of swimming pool water. Common anions, including CO32−, Ac−, I−, Br−, F−, Cl−, C2O42−, PO43−, ClO3−, SO42−, and IO4− were used. As shown in Fig. S9a and b (ESI†), the fluorescence of both CPs can also be obviously quenched by IO4− in swimming pool water. It is clear that the emission intensities of both 1 and 2 decrease with the increase of IO4− concentration (Fig. S9c and d, ESI†). The fluorescence intensity ratios (I0/I) of 1 and 2vs. IO4− concentration still show good linear relationships in the lower concentration range, and the Ksv values of 1 and 2 toward IO4− in swimming pool water were determined to be 1.48 × 104 and 2.43 × 104 M−1, respectively (Fig. S9e and f, ESI†). The LOD values of 1 and 2 toward IO4− in swimming pool water were determined to be 0.71 and 0.43 μM, respectively. The anti-interference experiments indicate that 1 and 2 can selectively detect IO4− in the presence of other anions in swimming pool water (Fig. S9g and h, ESI†). The high selectivity, sensitivity and good linear correlation suggest that 1 and 2 can be applied as potential luminescent sensors in practical applications.
3.8 Detection of agricultural chemicals
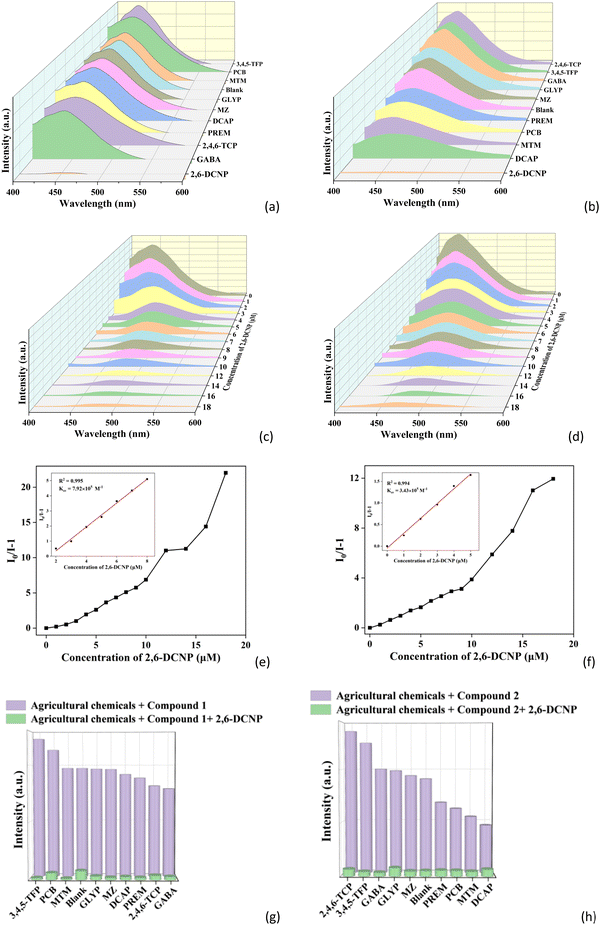
Since agricultural chemicals are organic substances, their solubility in water is poor, so EtOH was used as the solvent for better detection of agricultural chemicals. Through a similar approach to that for detection of inorganic anions, the luminescence spectra of 1 and 2 in the EtOH solutions of ten kinds of agricultural chemicals (2,4,6-trichlorophenol (2,4,6-TCP), 3,4,5-trifluorophenol (3,4,5-TFP), 2,6-dichloro-4-nitrophenol (2,6-DCNP), chlorobenzene (PCB), imidazole (MZ), methyltin mercaptide (MTM), glycine methyl ester hydrochloride (GABA), permethrin (PREM), 4-amino-2,6-dichlorophenol (DCAP), and N-(phosphonomethyl)glycine (GLYP)) with a concentration of 20 μM were investigated. From Fig. 5a and b, it can be clearly seen that 2,6-DCNP shows a significant quenching effect on the fluorescence intensity of both 1 and 2. However, other agricultural chemicals display negligible influence on the fluorescence intensity of 1 and 2 (Fig. S10a and b, ESI†), which also can be recognized by the naked eye (Fig. S10c and d, ESI†). These results suggest that 1 and 2 can efficiently sense 2,6-DCNP by luminescence quenching.The luminescence response of 1 and 2 was further investigated with different concentrations of 2,6-DCNP from 0 to 18 μM. As shown in Fig. 5c and d, the fluorescence intensity of 1 and 2 quickly decreases with increasing concentrations of 2,6-DCNP. The Ksv values of 1 and 2 toward 2,6-DCNP are 9.92 × 105 and 3.43 × 105 M−1 (Fig. 5e and f), respectively. The LOD values of 1 and 2 toward 2,6-DCNP are calculated to be 13.26 and 30.61 nM. Moreover, the anti-interference ability of 1 and 2 for the detection of 2,6-DCNP was studied. As shown in Fig. 5g and h, the fluorescence intensities of 1 and 2 both show an apparent decrease upon the addition of 2,6-DCNP, demonstrating that 1 and 2 can selectively detect 2,6-DCNP in the presence of other agricultural chemicals (Fig. S10e and f, ESI†). Such low LOD values and good anti-interference ability suggest that 1 and 2 have high sensitivity and selectivity to be luminescent sensors for detection of 2,6-DCNP. It is worth noting that no CP-based luminescent sensors of 2,6-DCNP have been reported so far, and CPs 1 and 2 are the first two examples of CP-based luminescent sensors of 2,6-DCNP.
In addition, CPs 1 and 2 were used to detect 2,6-DCNP in real farmland sewage. 3 mg ground samples of 1 and 2 were added into 2 mL treated farmland sewage and 1 mL EtOH and sonicated for 0.5 h, and then the stable suspensions of 1 and 2 in farmland sewage were obtained and used for the sensing experiments. By using a similar method to that in water described above, the common agricultural chemicals in farmland sewage, including 2,4,6-TCP, 3,4,5-TFP, 2,6-DCNP, PCB, MZ, MTM, GABA, PREM, DCAP, and GLYP, were used. As shown in Fig. S11a and b (ESI†), the fluorescence of both CPs can also be obviously quenched by 2,6-DCNP in farmland sewage. The concentration gradient experiments in farmland sewage (Fig. S11c and d, ESI†) reveal that the fluorescence intensities of both 1 and 2 decrease with increasing concentration of 2,6-DCNP. The Ksv values of 1 and 2 toward 2,6-DCNP are obtained as 2.19 × 105 and 2.02 × 105 M−1 (Fig. S11e and f, ESI†), respectively. The LOD values of 1 and 2 toward 2,6-DCNP in farmland sewage are calculated as 47.95 and 51.98 nM, respectively. The anti-interference experiments indicate that 1 and 2 can selectively detect 2,6-DCNP in the presence of other agricultural chemicals in farmland sewage (Fig. S11g and h, ESI†). These results suggest 1 and 2 can both be used as luminescent sensors for detection of 2,6-DCNP in real samples.
3.9 Detection of folic acid
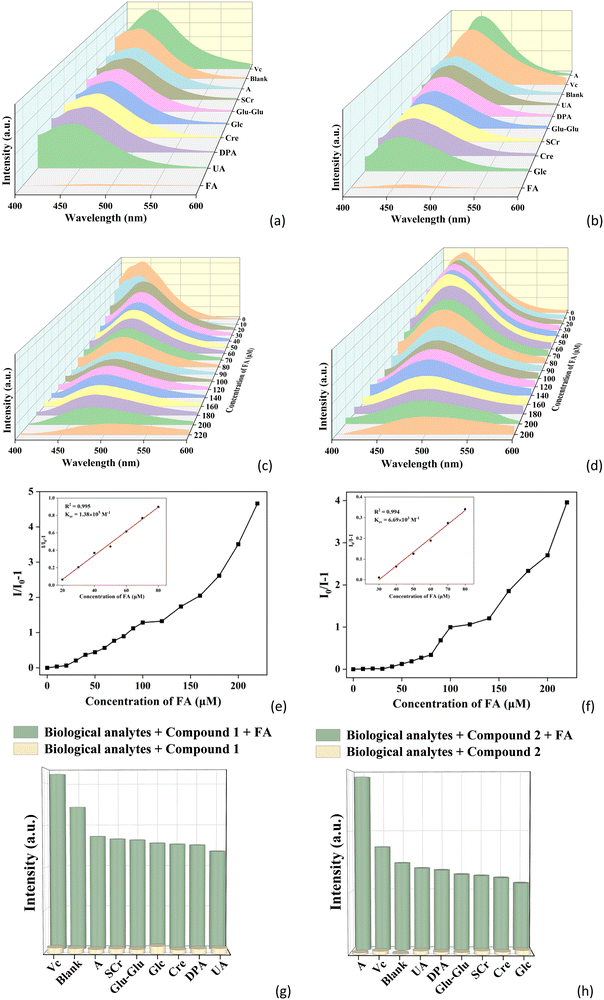
In view of the important roles of folic acid (FA) in the biochemical processes in the human body, herein 1 and 2 were also explored as luminescent sensors for detection of FA. Using a similar method as that in the detection of inorganic anions above, the luminescence spectra of 1 and 2 within the aqueous solutions of nine kinds of biological analytes (folic acid (FA), glucose (Glc), uric acid (UA), creatinine (SCr), creatine (Cre), vitamin C (Vc), L-alpha-glutamyl-L-glutamate (Glu–Glu), dipicolinic acid (DPA) and adenine (A)) with the concentration of 0.5 mM were recorded. As shown in Fig. 6a and b, FA shows the most remarkable quenching effect on the fluorescence intensities of 1 and 2, while the influences on the emission intensities of other biological substances are ignorable (Fig. S12a and b, ESI†). This also can be seen by the naked eye (Fig. S12c and d, ESI†). This implies that 1 and 2 can be applied as luminescent sensors to selectively detect FA in water.To determine the detection limits of 1 and 2 toward FA, concentration-dependent fluorescence sensing experiments were performed. As the concentration of FA increases from 0 to 220 μM, the fluorescence intensities of 1 and 2 gradually decrease (Fig. 6c and d). A good linear relationship can be observed at lower concentrations (Fig. 6e and f). The Ksv values of 1 and 2 toward FA are 1.38 × 104 and 6.69 × 103 M−1, respectively. The detection limits of 1 and 2 for FA are 13.04 and 15.70 μM, respectively, which are comparable with other reported fluorescent sensors of FA (Table S5, ESI†).63–67 The anti-interference experiments were further studied (Fig. 6g and h). Interestingly, it can be noted that the fluorescence responses of 1 and 2 toward FA were almost unaffected by the addition of other biological substances (Fig. S12e and f, ESI†). The above experimental results demonstrate that 1 and 2 have high sensitivity and excellent selectivity for the detection of FA.
To further verify the application of 1 and 2 in real samples for FA detection, the luminescence sensing experiments were carried out in human serum samples as using a similar method as that in water described above. The common components in serum, including lysine, leucine, glucose, Ca2+, Na+, Cl−, Zn2+, vitamin C, and FA were used in the sensing experiments. As shown in Fig. S13a and b (ESI†), the fluorescence of both CPs can also be obviously quenched by FA in serum. By performing concentration gradient experiments in serum (Fig. S13c and d, ESI†), the good linear relationship also can be observed at lower concentrations, with Ksv of 1 and 2 being 1.68 × 104 and 7.48 × 104 M−1, respectively (Fig. S13e and f, ESI†). The detection limits of 1 and 2 for FA are 0.63 and 0.14 μM. In the anti-interference experiments, it is clear that 1 and 2 also can selectively detect FA in the presence of other components of the serum (Fig. S13g and h, ESI†). All above experimental investigations indicate that 1 and 2 can be applied as luminescent sensors for selectively detect FA in real samples.
3.10 Sensing mechanism
According to the reported works, the possible mechanisms of fluorescence quenching are structural collapse (SC), competitive absorption (CA), photoinduced electron transfer (PET) and fluorescence resonance energy transfer (FRET).68,69 To investigate the quenching mechanisms of 1 and 2 for the detection of IO4−, 2,6-DCNP and FA, a series of experiments were performed. Firstly, the PXRD patterns of 1 and 2 after detecting IO4−, 2,6-DCNP and FA were measured. As shown in Fig. S14 (ESI†), the diffraction positions have no significant changes, indicating that the fluorescence quenching was not caused by the collapse of the structures of 1 and 2. Then, the IR spectra of 1 and 2 soaked in solutions of IO4−, 2,6-DCNP and FA were recorded. It can be seen from Fig. S15 (ESI†) that the absorption peaks remain unchanged compared with those observed before the sensing experiments. Thus, it is also further confirmed that the frameworks of 1 and 2 are intact without collapse. In addition, the UV-vis absorption spectra were measured. As illustrated in Fig. S16 (ESI†), the UV-vis spectra of IO4−, 2,6-DCNP and FA all have some overlap with the excitation spectra of 1 and 2, while there is no overlap with the emission spectra of two CPs. Thus the excitation light of two CPs can be absorbed by IO4−, 2,6-DCNP and FA, which ultimately results in the fluorescence quenching. This suggests that the competitive absorption is an important reason for the fluorescence quenching effects toward IO4−, 2,6-DCNP and FA. Moreover, the fluorescence lifetimes of 1 and 2 before and after soaking in solutions of IO4−, 2,6-DCNP and FA were also tested. As seen from Fig. S17 (ESI†), the fluorescence lifetimes of 1 and 2 are almost unchanged by the addition of IO4−, 2,6-DCNP, and FA, which indicates that the fluorescence quenching of 1 and 2 toward IO4−, 2,6-DCNP, and FA are static quenching processes. Therefore, the fluorescence quenching mechanism of 1 and 2 toward IO4−, 2,6-DCNP and FA should be due to competitive absorption and static quenching, which are similar to other previously reported CP-based sensors.70–723.11 Recyclable detection
Recyclable detection is an important evaluation indicator for practical applications. The suspensions of 1 and 2 were centrifuged after fluorescence sensing experiments, washed three times with distilled water and then dried and subjected to fluorescence sensing again, which was repeated for a total of five rounds. As shown in Fig. S18 (ESI†), the fluorescence intensities of 1 and 2 display no obvious decrease after five cycles. The PXRD patterns after five cycles also remain unchanged (Fig. S19, ESI†), which demonstrates that both CPs still maintain structural stability after five cycles. These results indicate that 1 and 2 have excellent recyclability for the detection of IO4−, 2,6-DCNP and FA.3.12 Films for visual inspection
By blending CPs with polymer materials, sensing films with fluorescence response can be prepared. Such films can realize detection and monitoring of specific substances by changes in luminescence intensity. For example, sensing films for detecting harmful substances in the environment can be prepared by blending CPs with a polymer material. When harmful substances are present, the CPs in the films undergo fluorescence quenching or fluorescence enhancement, resulting in obvious color changes or luminescence signals to achieve visual detection of harmful substances. Based on this, the ground powder samples of 1 and 2 were mixed with the polymer material poly (methyl methacrylate) (PMMA) in DMF and heated to form suspensions. The suspensions were uniformly dispensed in two surface dishes and held in an oven at 60 °C for 10 hours to obtain dried 1@PMMA and 2@PMMA films. Each film was cut into four 1 × 2 cm strips, and then IO4−, 2,6-DCNP and FA were added dropwise to the strip films under the irradiation of UV lamps. It was found that the films with addition of IO4−, 2,6-DCNP and FA emitted a darker light than those of the blank films (Fig. S20, ESI†). Based on the above experiments, it demonstrated that 1@PMMA and 2@PMMA can be used as visualized fluorescence sensing films for the naked eye detection of IO4−, 2,6-DCNP and FA.4. Conclusions
In summary, two novel Ni(II)/Zn(II)-based CPs were designed and synthesized using H2seb and the π-rich bbimh ligand under hydrothermal conditions. The two CPs present two distinct structures for the different coordination behaviors of metal centers and ligands. Fluorescence sensing experiments show that they exhibit high selectivity and sensitivity, excellent anti-interference and recyclability for the detection of IO4−, 2,6-DCNP and FA by fluorescence quenching. The two CPs also proved to be excellent sensors for the detection of IO4− in swimming pool water, 2,6-DCNP in farmland sewage and FA in human serum, respectively. Further studies confirm that competitive absorption and static quenching processes are the possible mechanisms for the fluorescence quenching. In addition, portable fluorescent films were prepared for rapid and convenient detection of IO4−, 2,6-DCNP and FA, which further expands their practical applications. This work provides new ideas for the design and development of luminescent CPs as potential sensing materials, which further expands the application of CPs in the biological and environmental fields.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for compounds 1 and 2 have been deposited at the CCDC with No. 2348596–2348597.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21601105) and the Natural Science Foundation of Shandong Province (ZR2022MB070).Notes and references
- M. Qian, L. Zhang, Z. Pu, C. Zhang, Q. Chen, X. Sui, X. Han, S. Zeng, H. Cui, J. Wang and X. Peng, Sens. Actuators, B, 2021, 344, 130764 CrossRef
.
- W. Wang, L. Lu, K.-J. Wu, J. Liu, C.-H. Leung, C.-Y. Wong and D.-L. Ma, Sens. Actuators, B, 2019, 288, 392–398 CrossRef CAS
.
- K. I. Hong, W. H. Choi and W. D. Jang, Dyes Pigm., 2019, 162, 984–989 CrossRef CAS
.
- A. Salimi, A. Noorbakhash and F. S. Karonian, Int. J. Electrochem. Sci., 2006, 1, 435–445 CrossRef CAS
.
- V. Romero, V. Vila, I. de la Calle, I. Lavilla and C. Bendicho, Sens. Actuators, B, 2019, 280, 290–297 CrossRef CAS
.
- Y. Wu, D. Qin, Z. Luo, S. Meng, G. Mo, X. Jiang and B. Deng, ACS Sustainable Chem. Eng., 2022, 10, 5195–5202 CrossRef CAS
.
- J. Min, L. Xu, S. Fang, W. Chen and X. Hu, Environ. Pollut., 2020, 258, 113703 CrossRef CAS
.
- J. Xiao, S. Zhu, L. Bu, Y. Chen, R. Wu and S. Zhou, RSC Adv., 2023, 13, 27203–27211 RSC
.
- Z. Ke, M. Lan, T. Yang, W. Jia, Z. Gou, K. Chen and J. Jiang, Environ. Res., 2021, 198, 111216 CrossRef CAS
.
- P. K. Arora, A. Srivastava, S. K. Garg and V. P. Singh, Bioresour. Technol., 2018, 250, 902–909 CrossRef CAS
.
- B. Yang, X. Li, L. Wang, J. An, T. Wang, F. Zhang, B. Ding and Y. Li, Talanta, 2020, 217, 121019 Search PubMed
.
- A. Sreekumar, L. Durai and S. Badhulika, New J. Chem., 2023, 47, 8845–8853 RSC
.
- R. Nakum, A. K. Ghosh, B. Ranjan Jali and S. K. Sahoo, Spectrochim. Acta, Part A, 2024, 313, 124143 CrossRef CAS
.
- H. A. Silvaneto, P. J. S. Barbeira, W. K. T. Coltro and E. Piccin, Food Chem., 2024, 444, 138677 CrossRef CAS
.
- J. Z. Liu, Y. B. Fu, N. Yang, Q. L. Wen, R. Sheng Li, J. Ling and Q. Cao, Spectrochim. Acta, Part A, 2024, 306, 123586 CrossRef CAS PubMed
.
- J. Liu, J. Liu, W. Liu, H. Zhang, Z. Yang, B. Wang, F. Chen and H. Chen, Inorg. Chem., 2015, 54, 7725–7734 Search PubMed
.
- X. Xie, Y. Hu, C. Zhang, J. Song, S. Zhuang and Y. Wang, RSC Adv., 2019, 9, 301–306 Search PubMed
.
- F. Liu, C. Xu, J. Li, Z. Zhang, X. Jin and B. Wang, J. Mol. Struct., 2023, 1294, 136499 CrossRef CAS
.
- I. A. Mir, K. Rawat, P. R. Solanki and H. B. Bohidar, J. Nanopart. Res., 2017, 19, 260 Search PubMed
.
- S. Yan, D. Deng, L. Zhang and Y. Lv, Talanta, 2019, 201, 96–103 CrossRef CAS
.
- S. Mukherjee, S. Ghosh and S. Biswas, Inorg. Chem. Front., 2022, 9, 6288–6298 RSC
.
- X. Zhao, J. Wu and W. Tian, CrystEngComm, 2023, 25, 945–952 Search PubMed
.
- Y. F. Jing, D. J. Young, Q. Huang, Y. Mi, S. C. Zhang and F. L. Hu, Spectrochim. Acta, Part A, 2020, 238, 118443 CrossRef CAS
.
- J. Suebphanpho and J. Boonmak, RSC Adv., 2024, 14, 9781–9790 RSC
.
- W. M. Wang, R. R. Cheng, Z. L. Wu and J. Z. Cui, Inorg. Chem., 2023, 62, 14902–14911 CrossRef CAS PubMed
.
- S. Mondal, R. Sahoo and M. C. Das, Inorg. Chem., 2023, 62, 14124–14133 CrossRef CAS
.
- N. A. Ashashi, C. Sen, M. Ahmad, A. K. Jassal and H. N. Sheikh, J. Solid State Chem., 2024, 334, 124659 Search PubMed
.
- S. Zhu, Z. Tan, J. Pan, L. Liu, T. Yang, X. Zhou, Y. You and H. Xiao, Eur. J. Inorg. Chem., 2024, e202300711 CrossRef CAS
.
- B. Li, Q. Q. Yan and G. P. Yong, J. Mater. Chem. C, 2020, 8, 11786–11795 RSC
.
- L. Zhai, Z. X. Yang, W. W. Zhang, J. L. Zuo and X. M. Ren, J. Mater. Chem. C, 2018, 6, 7030–7041 RSC
.
- S. Singh, M. K. Ghorai and K. K. Kar, J. Mater. Chem. A, 2023, 11, 8003–8012 RSC
.
- S. Xiang, J. Liu, G. Han, W. Zhang, Y. Long, Y. Deng, B. Wang and Q. Weng, Chem. Eng. J., 2023, 470, 144177 CrossRef CAS
.
- M. R. Yin, Q. Q. Yan, B. Li and G. P. Yong, CrystEngComm, 2021, 23, 3196–3203 RSC
.
- C. Xu, M. M. Sheng, H. T. Shi, M. Stromme and Q. F. Zhang, Dalton Trans., 2019, 48, 5505–5510 RSC
.
- S. Li, B. Wang, G. Liu, X. Li, C. Sun, Z. Zhang and X. Wang, Inorg. Chem. Front., 2024, 11, 1561–1572 Search PubMed
.
- J. X. Li, Y. J. Lu, K. Y. Quan, L. B. Wu, X. Feng and W. Z. Wang, J. Mol. Struct., 2024, 1297, 136830 Search PubMed
.
- M. Parsaei, K. Akhbari and J. White, J. Mol. Struct., 2023, 1283, 15224 CrossRef
.
- S. Bhunia, D. Sahoo, S. Maity, B. Dutta, S. Bera, N. B. Manik and C. Sinha, Inorg. Chem., 2023, 62, 11976–11989 CrossRef CAS
.
- Y. N. Wang, H. Xu, S. D. Wang, J. T. Bai, Q. C. Qiu, Y. Mo, W. Y. Feng, M. H. Zhang, Y. T. Wang, X. P. Chang and Q. F. Yang, J. Mol. Struct., 2024, 1298, 137119 CrossRef CAS
.
- P. Y. Du, W. P. Lustig, S. J. Teat, W. Gu, X. Liu and J. Li, Chem. Commun., 2018, 54, 8088–8091 Search PubMed
.
- H. Zhang, M. Sun, Y. Wang, L. Yin, D.-L. Ma, C.-H. Leung and L. Lu, J. Mater. Chem. B, 2022, 10, 1853–1857 RSC
.
- A. M. Somnath and K. A. Siddiqui, Dalton Trans., 2023, 52, 3643–3660 RSC
.
- R. Luo, C. G. Xu, D. M. Zhang, L. L. Wang, R. X. Wu, G. B. Chen, P. Lu, Y. H. Fan and F. Shao, Talanta, 2023, 265, 124803 CrossRef CAS PubMed
.
- J. J. Zhao, L. Zhang, P. Y. Liu, W. Z. Chen, Z. L. Liu and Y. Q. Wang, Dalton Trans., 2021, 50, 553–561 Search PubMed
.
- J. X. Hu, X. F. Jiang, Y. J. Ma, X. R. Liu, B. D. Ge, A. N. Wang, Q. Wei and G. M. Wang, Sci. China: Chem., 2021, 64, 432–438 Search PubMed
.
- D. X. Feng, Y. Mu, J. Li, S. De Han, J. H. Li, H. L. Sun, M. Pan, J. X. Hu and G. M. Wang, Adv. Funct. Mater., 2023, 33, 2305796 CrossRef CAS
.
- Z. H. Zhao, F. H. Zhao, Z. L. Li, Y. S. Li, R. Feng, J. M. You and Y. C. He, J. Mol. Struct., 2024, 1305, 137821 CrossRef CAS
.
- F. H. Zhao, Z. H. Zhao, Y. S. Li, R. Feng, T. Han, Y. C. He and Z. L. Li, J. Mol. Struct., 2024, 1298, 137051 CrossRef CAS
.
- L. M. Pu, L. L. Gan, Y. N. Yue, G. H. Liu, W. B. Xu, H. T. Long and W. K. Dong, J. Mol. Struct., 2024, 1308, 138024 CrossRef CAS
.
- F. H. Zhao, Z. L. Li, Y. C. He, L. W. Huang, X. M. Jia, X. Q. Yan, Y. F. Wang and J. M. You, J. Solid State Chem., 2019, 271, 309–313 Search PubMed
.
- R. H. Ismayilov, F. F. Valiyev, D. B. Tagiyev, Y. Song, A. A. Medjidov, P. A. Fatullayeva, B. Tüzün, P. Taslimi, C. H. Peng, S. Y. Chien, G. H. Lee and S. M. Peng, J. Mol. Struct., 2024, 1307, 137966 CrossRef CAS
.
- J. Song, M. Wu, Y. L. Zhou, L. X. Jin, Y. H. Gao and H. P. Dai, J. Mol. Struct., 2024, 1307, 137955 CrossRef CAS
.
- Z. Q. Huang, J. Q. Chen, X. Y. Zhang, C. K. Yuan, P. Wang, Y. Zhao, B. C. Zhao, W. X. Qi and W. Y. Sun, CrystEngComm, 2024, 26, 1701–1709 RSC
.
- K. Zhang, Q. Wang, Y. Gong, N. Wang and X. Li, CrystEngComm, 2023, 25, 3033–3043 RSC
.
- H. Zhu, C. Han, Y. H. Li and G. H. Cui, J. Solid State Chem., 2020, 282, 121132 Search PubMed
.
- H. Zhu, D. Liu, Y. H. Li and G. H. Cui, Polyhedron, 2019, 167, 44–50 Search PubMed
.
- Z. C. Hao, S. C. Wang, Y. J. Yang and G. H. Cui, Polyhedron, 2020, 181, 114466 Search PubMed
.
- M.-H. Yan, J. Wang, X.-Y. Su, H. Sakiyama, N. Qi, M. Afzal, A. Alarifi, D. Srivastava and A. Kumar, New J. Chem., 2023, 47, 11134–11142 RSC
.
- F. H. Zhao, Y. S. Li, R. Feng, Z. H. Zhao and Z. L. Li, J. Inorg. Organomet. P., 2023, 34, 969–978 Search PubMed
.
- Z. F. Wu, Z. H. Fu, E. Velasco, K. Xing, H. Wang, G. D. Zou, X. Y. Huang and J. Li, J. Mater. Chem. C, 2020, 8, 16784–16789 Search PubMed
.
- J. Zhang, L. Fan, Y. Zhao, C. Sun, W. Li and Z. Chang, Inorg. Chim. Acta, 2023, 547, 121330 CrossRef CAS
.
- S. Zhu, Q. Wang, X. Wang, J. Pan, T. Yang, X. Zhou, H. Xiao and Y. You, Inorg. Chem., 2023, 62, 16589–16598 CrossRef CAS
.
- D. Li, Y. Jia, Z. Li, L. Liu, N. Wu and M. Hu, Dalton Trans., 2023, 52, 696–702 RSC
.
- Y. Jiang, Y. Huang, X. Shi, Z. Lu, J. Ren, Z. Wang, J. Xu, Y. Fan and L. Wang, Inorg. Chem. Front., 2021, 8, 4924–4932 RSC
.
- Z. P. Dong, F. Zhao, L. Zhang, Z. L. Liu and Y. Q. Wang, New J. Chem., 2020, 44, 10239–10249 RSC
.
- K. Wang, Z. Y. Li, Y. Peng, T. F. Zheng, J. L. Chen, S. J. Liu and H. R. Wen, Inorg. Chem., 2023, 62, 17993–18001 CrossRef CAS
.
- D. Li, Y. Yang, S. Su, Y. Jia, H. Xing and M. Hu, Microchem. J., 2024, 199, 109995 Search PubMed
.
- X. Sun, C. Li, X. Meng, D. Wang and C. Zheng, CrystEngComm, 2023, 25, 4531–4538 RSC
.
- Y. Zhao, H. Zeng, X. W. Zhu, W. Lu and D. Li, Chem. Soc. Rev., 2021, 50, 4484–4513 RSC
.
- Y. Wang, N. Xu, J. Ma, H. Li, Y. Zhang, G. Liu and X. Wang, Inorg. Chem., 2022, 61, 7780–7786 CrossRef CAS PubMed
.
- X. Wang, Y. Li, Z. Tan, L. Liu, Y. H. Li, X. Zhou, Y. You, S. Wang and H. P. Xiao, Cryst. Growth Des., 2023, 23, 6851–6859 CrossRef CAS
.
- H. Y. Yin, Q. Li, T. H. Liu, J. Liu, Y. T. Qin, Y. Wang, W. L. Zhai, X. B. Cai, Z. G. Wang and W. Zhu, Inorg. Chem., 2024, 63, 1816–1827 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2348596 and 2348597. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4tc01686f |
| This journal is © The Royal Society of Chemistry 2024 |