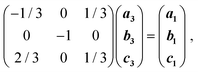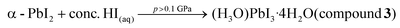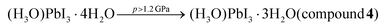High-pressure observation of elusive iodoplumbic acid in different hydronium-hydrate solid forms†‡
Szymon
Sobczak
 a,
Athena M.
Fidelli
b,
Jean-Louis
Do
a,
Athena M.
Fidelli
b,
Jean-Louis
Do
 b,
George P.
Demopoulos
b,
George P.
Demopoulos
 *c,
Audrey
Moores
*c,
Audrey
Moores
 *b,
Tomislav
Friščić
*b,
Tomislav
Friščić
 *bd and
Andrzej
Katrusiak
*bd and
Andrzej
Katrusiak
 *a
*a
aDepartment of Materials Chemistry, Faculty of Chemistry, Adam Mickiewicz University, Uniwersytetu Poznanskiego 8, 61-614 Poznań, Poland. E-mail: katran@amu.edu.pl; Web: https://twitter.com/szymon_sobczak Web: https://twitter.com/DMCh_AMU
bDepartment of Chemistry, McGill University, 801 Sherbrooke St West, Montreal, QC H3H 0B8, Canada. E-mail: audrey.moores@mcgill.ca; Web: https://twitter.com/MooresResearch Web: https://twitter.com/JLD_Chemist Web: https://twitter.com/TomislavFriscic
cDepartment of Materials Engineering, McGill University, 801 Sherbrooke St West, Montreal, QC H3A 0C5, Canada. E-mail: george.demopoulos@mcgill.ca
dSchool of Chemistry, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK. E-mail: t.friscic@bham.ac.uk
First published on 6th December 2023
Abstract
High-pressure and high-temperature isochoric crystallization combined with single-crystal X-ray diffraction revealed the proposed, but previously never demonstrated, hydronium acid hydrates of [PbI3]−, the closest forms of the elusive iodoplumbic acid. Depending on the pressure range, the reaction of PbI2 and aqueous concentrated hydriodic acid under isochoric conditions in a diamond anvil cell held between 0.11 and 1.20 GPa produces two hydrated acids with compositions [H3O][PbI3]·nH2O (n = 3, 4). Comprised of polymeric one-dimensional double-chain PbI3− anions, analogous to those seen in archetypal lead-halide perovskites such as RbPbI3 and CsPbI3, these hydrates offer the first observation of the iodoplumbic acid progenitor of hybrid lead perovskites. We also reveal the ‘hidden’ polymorph of lead(II) iodide, adopting a three-dimensional Pb–I bonded network, contrasting with the prototypic ambient-pressure layered PbI2 structure.
Introduction
The emergence of photovoltaic lead halide perovskites APbI3, where A = alkaline metal, ammonium or organoammonium cation has sparked extensive studies on the existence of iodoplumbic(II) acid, HPbI3.1,2 In most cases, lead halide perovskites are composed of polymeric PbI3− anions, such as NH4PbI3,3 [CH3NH3]PbI3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4–9 or CsPbI3,10–12 which can be synthesized via traditional13 as well as unconventional methods.14–16 The proposed HPbI3 would be the simplest member of this compound class and the progenitor of hybrid, as well as inorganic lead(II) perovskites. There were several attempts to propose a computational prediction of the most probable HPbI3 structure, which suggested that it forms a 3-dimensional perovskite structure of space-group symmetry Pm
4–9 or CsPbI3,10–12 which can be synthesized via traditional13 as well as unconventional methods.14–16 The proposed HPbI3 would be the simplest member of this compound class and the progenitor of hybrid, as well as inorganic lead(II) perovskites. There were several attempts to propose a computational prediction of the most probable HPbI3 structure, which suggested that it forms a 3-dimensional perovskite structure of space-group symmetry Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, consisting of the PbI3-polyanionic framework encapsulating hydrogen inside the cubic cages.17 Whereas iodoplumbic acid was proposed as a precursor for the synthesis of hybrid perovskites in 2015 by Zhao et al.,18 the existence of an acid with composition HPbI3 has remained controversial.19–28 The groups of Kanatzidis and of Hillebrecht29,30 have shown that the postulated HPbI3 precipitate obtained from N,N-dimethylformamide (DMF) solutions of PbI2 and hydriodic acid (HI) is actually the dimethylammonium hybrid perovskite [N(CH3)2H2]PbI3. Moreover, Daub and Hillebrecht demonstrated that the reaction of PbI2 with concentrated (57% w/w) aqueous HI can produce two forms of hydrated iodoplumbic acid.30 One form exhibits the composition [H3O]2x[Pb1−xI2]·(2 − 2x)H2O (1) (x ≈ 0.23), and is based on two-dimensional (2-D) anionic CdI2-type sheets with approximate composition [Pb3I82−]n (Fig. 1a). The second reported form of hydrated iodoplumbic acid exhibits the composition (H3O)2Pb3I8·6H2O (2) (Fig. 1b), and is based on one-dimensional (1-D) polyanionic tapes of [Pb3I82−]n.
m, consisting of the PbI3-polyanionic framework encapsulating hydrogen inside the cubic cages.17 Whereas iodoplumbic acid was proposed as a precursor for the synthesis of hybrid perovskites in 2015 by Zhao et al.,18 the existence of an acid with composition HPbI3 has remained controversial.19–28 The groups of Kanatzidis and of Hillebrecht29,30 have shown that the postulated HPbI3 precipitate obtained from N,N-dimethylformamide (DMF) solutions of PbI2 and hydriodic acid (HI) is actually the dimethylammonium hybrid perovskite [N(CH3)2H2]PbI3. Moreover, Daub and Hillebrecht demonstrated that the reaction of PbI2 with concentrated (57% w/w) aqueous HI can produce two forms of hydrated iodoplumbic acid.30 One form exhibits the composition [H3O]2x[Pb1−xI2]·(2 − 2x)H2O (1) (x ≈ 0.23), and is based on two-dimensional (2-D) anionic CdI2-type sheets with approximate composition [Pb3I82−]n (Fig. 1a). The second reported form of hydrated iodoplumbic acid exhibits the composition (H3O)2Pb3I8·6H2O (2) (Fig. 1b), and is based on one-dimensional (1-D) polyanionic tapes of [Pb3I82−]n.
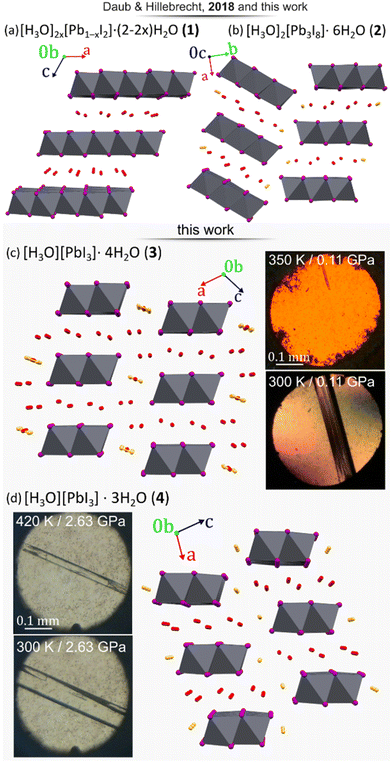 | ||
| Fig. 1 Four forms of hydrated iodoplumbic acid obtained by reaction of PbI2 and concentrated aqueous HI: (a) [H3O]2x[Pb1−xI2]·(2 − 2x)H2O (1, x ≈ 0.23); (b) (H3O)2Pb3I8·6H2O (2);30 as well as new materials herein synthesized under high-pressure (p) and -temperature (T) conditions: (c) (H3O)PbI3·4H2O (3) and (d) (H3O)PbI3·3H2O (4). The proposed sites of H3O+ cations are marked in orange. Photographs show crystals 3 and 4in situ grown under pressure. | ||
Compound 2 was reported to be the first product of either crystallization of PbI2 from concentrated aqueous HI, or of the gas–solid reaction between PbI2 and HI vapours. When exposed to open air, 2 quickly transforms into 1. Anions in both 1 and 2 are separated by layers of water molecules containing hydronium ions. Overall, these prior studies suggest that an inorganic acid based on the PbI3− anion does not exist, and that the only accessible forms of iodoplumbic acid are hydronium salts of the Pb3I82− anion.
We now report the synthesis and observation of a hydronium salt of [Pb2I6]2− achieved under unconventional, isochoric conditions of elevated temperature and pressure. Specifically, we show that crystallization of PbI2 from concentrated aqueous HI provides, at pressure above 0.11 GPa, access to hydronium of 1-D double-chain polyanions with composition PbI3−. Based on the chemical and structural composition of the anion, which are identical to those in RbPbI3, CsPbI3etc., and the absence of any ammonium or metal cations in the system, the herein reported structures can be considered a significant step towards the observation and understanding of iodoplumbic acid, a historically elusive entity.
Results and discussion
With HPbI3 apparently inaccessible by solution techniques, our study has focused on less conventional reaction environments based on introduction of mechanical energy in the form of either ball milling (mechanochemistry)31–33 or by high-pressure chemistry. Our first exploration of reactivity between PbI2 and concentrated aqueous hydroiodic acid HI(aq) was done mechanochemically, by milling of the two components in the stoichiometric ratio of PbI2/HI = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (corresponding to the 3
1 (corresponding to the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 stoichiometric ratio of Pb to I, respectively). Milling produced a bright yellow powder that, upon powder X-ray diffraction (PXRD) analysis, matched the previously reported 1 (see ESI†). Increasing the amount of HI(aq) in the milling reaction to produce a 1
8 stoichiometric ratio of Pb to I, respectively). Milling produced a bright yellow powder that, upon powder X-ray diffraction (PXRD) analysis, matched the previously reported 1 (see ESI†). Increasing the amount of HI(aq) in the milling reaction to produce a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 respective stoichiometric ratio of PbI2 and HI led to the formation of 2 in a pure form (see ESI†). Consistent with previous work,30 upon standing in air 2 slowly transforms into 1 and, subsequently, into the orange solid PbI2.
3 respective stoichiometric ratio of PbI2 and HI led to the formation of 2 in a pure form (see ESI†). Consistent with previous work,30 upon standing in air 2 slowly transforms into 1 and, subsequently, into the orange solid PbI2.
A different approach to introduce mechanical energy to a reaction is in the form of hydrostatic pressure in a diamond anvil cell (DAC),34–40 allowing the exploration of otherwise inaccessible thermodynamic coordinates and formation of new products.39–42 As a reactor, the DAC represents an almost perfect closed system confining the reaction to the volume of ∼0.02 mm3 between two diamond culets and a steel gasket.41 For each of the high-pressure reactions, a saturated solution of PbI2 in HI(aq) was loaded in the DAC and isothermally compressed at 297 K. At 0.11 GPa, a polycrystalline mass precipitated that, upon subsequent isochoric recrystallization led to a colorless elongated prism-shaped crystal suitable for structure determination by single-crystal X-ray diffraction (SCXRD). After increasing pressure to 0.20 GPa, the unit-cell dimensions were determined (see ESI Table S1†) and the next SCXRD measurement at 0.5 GPa revealed a novel hydrated hydronium salt of composition (H3O)PbI3·4H2O (3). The structure of 3 consists of 1-D anionic PbI3− tapes of edge-sharing PbI6-octahedra running along the crystallographic b-axis (Fig. 1c). The PbI6-octahedra in the PbI3− polyanion are distorted, exhibiting four different lengths of Pb–I bonds around each metal ion: 3.046(2), 3.174(6) (twice), 3.250(6) (twice), and 3.3663(19) Å. These Pb–I bond length distances are very similar to those observed in diguanidinium tetraiodoplumbate43 and other 6-coordinated lead(II) complexes (see ESI, Fig. S10†), confirming the retention of the Pb2+ oxidation state. Importantly, the PbI3− anionic tapes in 3 are identical to those in NH4CdCl3·2H2O, hybrid and lead iodide perovskites CsPbI3, RbPbI3, as well as related hydrates NH4PbI3·2H2O, CsPbI3·2H2O, and CH3NH3PbI3·H2O.44–49 The structure of the anion makes compound 3 the first example of a direct hydronium-based acid analogue of these well-known lead perovskite solids. Crystallographic parameters for 3 are distinct from those of previously reported301 or 2 (Fig. 1c and Table 1).
| Compound |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
3 | 4 | β-PbI2 |
|---|---|---|---|---|---|
| a The herein determined structure [H3O]2x[Pb1−xI2]·(2 − 2x)H2O, x = 0.20(2), is analogous to that previously reported30 with x = 0.23. | |||||
| Formula | [H3O]0.40[Pb0.6I2]·1.6H2O | (H3O)2Pb3I8·6H2O | (H3O)PbI3·4H2O | (H3O)PbI3·3H2O | PbI2 |
| P (GPa) | 0.0001 | 0.0001 | 0.5 | 2.63 | 2.05 |
| T (K) | 300 | 300 | 300 | 300 | 320 |
| Space group | C2/m | Pbam | I2/m | P21/m | C2/m |
| a (Å) | 7.8946(10) | 10.033(3) | 16.205(2) | 9.57(3) | 14.06(4) |
| b (Å) | 4.5598(3) | 30.126(7) | 4.5170(1) | 4.513(3) | 4.4560(12) |
| c (Å) | 11.1985(18) | 4.5610(10) | 17.184(14) | 12.98(11) | 10.540(6) |
| β (°) | 118.023(19) | — | 111.50(4) | 95.9(6) | 93.08(12) |
| V (Å3) | 355.859 | 1378.66(6) | 1170.3(10) | 558(5) | 654.9(17) |
| D calc (g cm−3) | 4.638 | 4.290 | 3.666 | 3.883 | 7.013 |
Each [PbI3]n− polyanion requires a counter hydronium cation for the charge balance. Although the strong scattering of X-rays by heavy lead and iodine atoms hinders the precise location of hydrogen atoms in X-ray diffraction, the location of oxygen atoms and their shortest distances can discriminate the corresponding water molecules and hydronium ions. It is known that the hydronium cations form with iodide anions strong, charge-assisted O⋯I bonds, significantly shorter that those involving water molecules. In 3, the [Pb2I6]2− polyanions are separated by tapes of hydrogen-bonded water molecules (Fig. 1c and 2b) connected into an extended honeycomb-like structure through O–H⋯O hydrogen bonds with O⋯O distances of 2.63(4), 2.68(6) and 2.79(7) Å. The tapes propagate in the crystallographic b-direction and are periodically interrupted by iodoplumbate(II) anions, forming I⋯O contacts that are significantly shorter than the 3.54 Å non-bonding distance expected from the sum of van der Waals radii50 of O (1.50 Å) and I (2.04 Å) atoms, indicating the formation of the charge-assisted O–H⋯I bonds. The structure of 3 also exhibits 1-D arrays of oxygen atoms (with O⋯O distances of 2.41(6) Å) separate from the hydrogen-bonded honeycomb tapes, which are located between pairs of polymeric PbI3− anions (Fig. 2b). At 0.50 GPa, these oxygen atoms are situated at notably short I⋯O distances of 3.2(2) Å, that are commensurate with charge-assisted51 O–H⋯I hydrogen bonding, tentatively indicating the location of H3O+ ions.
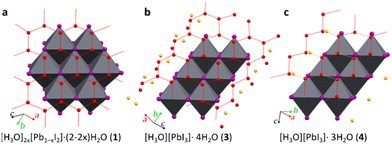 | ||
| Fig. 2 Fragments of crystal structures of (a) 1 herein re-determined30 from a crystal obtained by transformation of 3 at 0.1 MPa; (b) 3; and (c) 4, all viewed perpendicular to the honeycomb pattern of water molecules (red-dotted lines, orange-dashed lines in 4 mark the longest O⋯O distances). The oxygen-atom sites suggested for H3O+ ions are shown in orange. | ||
While increasing the pressure up to 1.20 GPa does not affect the crystals of 3, releasing the pressure to 0.1 MPa quickly leads to their transformation into 1 (Fig. 3). This chemical transformation takes place in a single-crystal-to-single-crystal manner, as shown by X-ray diffraction on the crystal recovered from the DAC (see ESI†), which revealed a clear matrix relationship between the lattices for the starting crystal structure of H3OPbI3·4H2O (3) and the daughter phase 1:
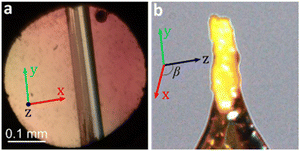 | ||
| Fig. 3 Single crystal of 3: (a) as grown in the DAC and (b) after being recovered to ambient conditions and transforming to 1, mounted on a nylon loop. Crystal axes are indicated. | ||
The quality of the crystal recovered to 0.1 MPa permitted the SCXRD measurement of lattice dimensions and structure refinement, which revealed monoclinic symmetry of space group C2/m (Table 1, Fig. 2a, also ESI†). The structure was found to be highly pseudo-symmetric (for details see ESI†) and similar to the trigonal structure previously reported for 1.30 The two determinations of this layered [H3O]2x[Pb1−xI2]·(2 − 2x)H2O structure are consistent (Fig. 1a and 2a), except for a somewhat lower x = 0.20(2) value resulting from our least-squares refinement, compared to x = 0.23.30 The difference, we believe, indicates the possibility of 1 to adopt a wider range of Pb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I stoichiometric compositions. The observed highly topotactic transformation52 from 3 to 1 requires a transition from 1-D to 2-D polyanions, in which some of the water and HI molecules leave the structure, probably by diffusion, while the edge-sharing connectivity of PbI6-octahedra is preserved in both materials.
I stoichiometric compositions. The observed highly topotactic transformation52 from 3 to 1 requires a transition from 1-D to 2-D polyanions, in which some of the water and HI molecules leave the structure, probably by diffusion, while the edge-sharing connectivity of PbI6-octahedra is preserved in both materials.
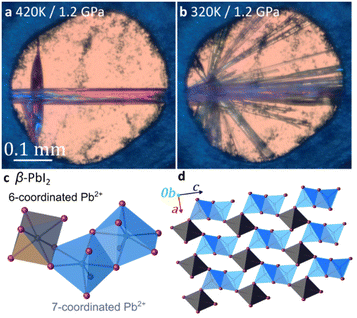
Above 1.2 GPa and above 420 K, a clearly distinct pink-coloured crystalline material (Fig. 4a and b) different from compound 3, is formed. Subsequent SCXRD at 320 K and pressures above 2.05 GPa revealed a ‘hidden’ polymorph of PbI2, herein termed β-PbI2. This novel form of β-PbI2 (Table 1, Fig. 4c, d, also ESI†) of the monoclinic space group C2/m and unprecedented for PbI2 polymorphs exhibits a 3-D framework of alternating six- and seven-coordinated Pb2+ cations bridged by iodide ions, in stark contrast to the well-known 2-D layered structure with six-coordinated Pb2+ cations herein termed α-PbI2.53,54 The β-PbI2 displays a rare property of reverse solubility, as we observed the growth of β-PbI2 crystals on increasing the temperature, and their dissolution on cooling of the DAC. The isochoric conditions in the DAC imply that the increased temperature results either in the increased pressure (for the overall positive thermal expansion of all components in the DAC chamber) or the pressure drops (for the overall negative thermal expansion). According to our knowledge on numerous high-pressure crystallizations reported in the literature, the temperature increase always enhanced dissolution of the solid compounds. There are very few compounds becoming less soluble with increasing temperature (e.g. Li2SO4); likewise, few compounds for which the reverse solubility with the increase of pressure were reported. The formation of β-PbI2 is consistent with high-pressure effect increasing the coordination numbers due to stronger compression of anions than cations.42,55,56 Recently, the high-pressure phases (phase II and III) of the 2H-type PbI2 polytype, first identified by Bridgman via isothermal compression,58,59 were characterized.57 Phase II (space group P3m1) crystallizes above 0.58 GPa as a two-dimensional 4H polytype, while in phase III (orthorhombic Pnma), stable above 2.6 GPa, each Pb atom is coordinated by 9 I-atoms similarly as in the orthorhombic PbCl2-structure.57 However, both phases II and III contrast with the complex structure of β-PbI2, which requires in situ recrystallization and cannot be obtained by cooling/heating or compressing another phase; hence β-PbI2 is referred to as a ‘hidden phase’. In this respect, PbI2 is similar to imidazole where the β phase can be accessed only by high-pressure recrystallization.60
Another crystalline phase (4) was obtained either by recrystallizing 3 above 1.2 GPa or by spontaneous recrystallization of β-PbI2 in the DAC below 350 K (Fig. 1d, also ESI†). Below 1.2 GPa, at 320 K, pink β-PbI2 dissolves and colourless needle-like crystals of 4 appear. Recrystallization by mild temperature oscillation produced a diffraction-quality single crystal of 4 (Fig. 1d and Table 1).
Compound 4 was found to exhibit the formula (H3O)PbI3·3H2O, again based on polymeric anions with edge-sharing PbI6-octahedra identical to those in 3 and a variety of lead halide perovskites, but with a lower content of crystallization water. The lower content of water in 4 compared to 3 is consistent with shorter I⋯I contacts at higher pressure. In the case of compound 4, also four distinct lengths of Pb–I bonds are present in the anions: 2.81(4), 3.186(12) (twice), 3.232(11) (twice), and 3.34(4) Å. These bond lengths are consistent with lead again adopting the Pb2+ oxidation state (Fig. S10†), implying the anion composition PbI3−.
In 4, oxygen atoms of water molecules form ribbons (about 10 Å wide). Within the ribbons shorter hydrogen bonds [O⋯O distances of 2.73(9) Å] are arranged into three 1-D zigzag chains, interconnected by weaker hydrogen bonds [O⋯O distance of 3.1(2) Å] into a strongly distorted honeycomb motif. The ribbons separate the adjacent pairs of PbI3− polyanions. Additionally, there are also oxygen atoms not involved in any O⋯O contacts commensurate with hydrogen bonds, but they are each close to iodine atoms of PbI3− anions, with O⋯I distances of 3.187(12) (twice), 3.232(11) (twice), 2.80(4) and 3.33(4) Å, at a pressure of 2.63 GPa and a temperature of 300 K. As indicated above, the hydronium cations and water molecules (Fig. 2c) were discriminated according to the O⋯PbI3− distances, shorter for the charge-assisted OH+⋯I bonds.
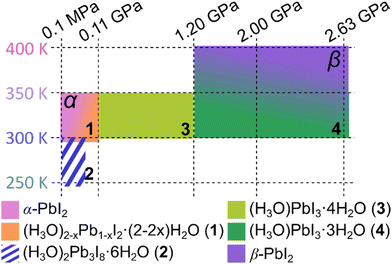
The appearance of the herein described series of structures comprising the α- and β-forms of PbI2, as well as hydrated iodoplumbic acids 1–4 can be rationalized through the interplay of effects related to the intercalation of HI(aq) and to high-pressure conditions. Specifically, the observation of different structures at different conditions outlines several stability regions in the preference p–T diagram (Fig. 5), where the low-pressure end-member is α-PbI2 with a 2-D layer structure, and the high-pressure end-member is β-PbI2, in which some of the Pb2+ cations become 7-coordinated to form a 3-D network.
All members of the series, exhibiting either 1-D, 2-D or 3-D structures, contain the common motif of edge-sharing PbI6− octahedra (Fig. 1 and 2), based on Pb–I bonds that are by far and large the least compressed elements constituting the scaffolds of the structures (Table 1). In contrast, the I⋯I contacts between PbI2 sheets or the iodoplumbate(II) anions are expected to be considerably weaker and most likely to be affected by pressure, temperature and overall chemical environment. The structures 1–4 can be seen as resulting from the α-PbI2 structure through intercalation of water and H3O+ from HI(aq), resulting in O–H⋯I bonds between polyanionic sheets and tapes, which prevent their contacts involving iodine atoms.
The observation of compounds 1–3 at pressures up to 1.2 GPa occurs by progressive insertion of hydronium ions from HI(aq) into the PbI2 structure, leading to the formation of an anion with PbI3− composition. At 1.2 GPa a partial desorption of water is observed, leading to the formation of compound 4 that maintains the structure and composition of the PbI3− anions. This chemical process first requires a dissolution of 3 and it is consistent with our previous general observation that recrystallizations conducted above 1 GPa often destabilize hydrates.60–62 Ultimately, exposing the system to still higher pressure and temperature completely prevents water intercalation into the structure of PbI2 and leads to the formation of a 3-D structure through creation of new Pb–I bonds and 7-coordinated lead(II) ions.
The effect of temperature and pressure on the aqueous solutions of α-PbI2 and HI can be represented in the chemical formula reported by Daub and Hillebrecht:30 (H2O)δ(PbI2)ε(HI)ζ.
According to this formula, the stoichiometry of compounds 1–4 can be represented as:
| Compound 1 (H2O)2.597(PbI2)(HI)0.597 for x = 0.23 [ref. 30] |
| Compound 1′ (H2O)2.5(PbI2)(HI)0.5 for x = 0.20 [this work] |
| Compound 2 (H2O)2.667(PbI2)(HI)0.667 [ref. 30 & this work] |
| Compound 3 (H2O)5(PbI2)(HI) [this work] |
| Compound 4 (H2O)4(PbI2)(HI) [this work], |
The herein observed structures of hydrated iodoplumbic acids 1–4 resemble some of those recently observed for solvates of popular methylammonium (CH3NH3+) and formamidinium (CH(NH2)2+) iodoplumbates with N,N-dimethylformamide (DMF), S,S-dimethylsulfoxide (DMSO) and/or water.49 Such solvates have attracted significant attention as intermediates in the formation,63–65 or products of moisture-induced degradation, of hybrid perovskite thin films highlighting the importance of the current findings.66,67 Specifically, structures containing polymeric Pb3I82− anions, reminiscent of the ones found in 2, were observed as solvates for both methylammonium and formamidinium iodoplumbate with DMF,68,69 and in the former case also in a solvate with DMSO.70 While the PbI3− anionic double-chain motifs observed in 3 and 4 are also found in several hydrates of alkaline metal iodoplumbates(II), it is also found in the hydrate and an alternative DMF solvate of CH3NH3PbI3.49,69 In these two structures, the anionic PbI3− double chains are aligned in parallel and separated by protonated cations and solvent molecules (water, DMF), and form arrangements broadly similar to the crystal structures of 3 and 4, where the anions also lie aligned in parallel and are separated by hydronium cations and water molecules. However, compounds 3 and 4 are not strictly isostructural to any of these previously reported solvated structures.
Other types of anions, not yet observed in our high-pressure studies, have been observed in solvated forms of methylammonium and formamidinium iodoplumbates, notably a dihydrate of the methylammonium salt based on monomeric octahedral PbI64− ions,71 as well as DMF72 and DMSO73 solvates of the methylammonium salts containing polymeric PbI5− anions composed of corner-sharing PbI6-octahedra. Importantly in the context of the present work, the crystal structure68 of the DMF solvate of CH(NH2)2PbI3 presents an alternative form of the PbI3− anion, in the form of simple chains formed by face-sharing PbI6-octahedra. While our studies have so far not revealed any evidence for such a phase based on hydronium ions, we believe they suggest the possibility of another, structurally distinct, class of iodoplumbic acid hydrates.
Conclusion
In summary, high-energy isochoric syntheses in a DAC revealed the first structures of the so far elusive and controversial iodoplumbic acid, in the form of a hydrated hydronium salts of [PbI3]− achievable at elevated temperature and pressure, providing new experimental information relevant for the previous speculations regarding possible HPbI3 structure. In that respect, the iodoplumbic acids synthesized under high-pressure resemble other inorganic acids that are known only in the hydrated from, such as HAuCl4 or HICl4.74,75 The herein obtained hydronium-based acids are based on 1-D double-chain of composition PbI3− anions, analogous to those found in the archetypal, well-known lead perovskites RbPbI3, CsPbI3, NH4PbI3·2H2O, or CsPbI3·2H2O.44–48 The composition and structure of anions distinguish these high-pressure materials from iodoplumbic acids made by recrystallization of PbI2 from aqueous hydriodic acid,30 and render them the so far first observed examples of iodoplumbic acid: a material of fundamental significance as the formal inorganic progenitor of the highly popular class of hybrid perovskite materials. The herein described series of hydrated iodoplumbic acids constitutes a family of closely related compounds with those previously reported at standard pressure,30 and their high-pressure synthesis can be described through the following equations:These processes can be rationalized through intercalation of water molecules and hydronium ions between negatively charged lead iodide fragments, resulting in O–H⋯I and O–H+⋯Iδ− hydrogen bonds that reduce electrostatic repulsion and prevent close contacts between the anions. This system exhibits high sensitivity to external stimuli, as evidenced by the observation of so far four iodoplumbic acids, up to high pressures (>2 GPa) and temperatures (>350 K) at which intercalation is no longer favoured and a new form of lead(II) iodide (β-PbI2) appears (see the equation above). The β-PbI2 structure is the unique polymorph of lead(II) iodide and, in contrast to the previously reported phases,57 it is based on the 3-dimensional network of edge-sharing PbI6 and PbI7 octahedra.
Overall, this work provides new fundamental chemical information about lead-iodide perovskite solids, viewed from the perspective of high-pressure and high-temperature conditions. The role of investigated PbI2/HI(aq) system as a key intermediate in the synthesis of perovskite-based solar cells and its use as a model for probing novel perovskite materials can unveil novel pathways in the renewable energy industry. This exploration underscores the criticality of further research into iodoplumbic-based materials applications, potentially catalysing breakthroughs in the design and performance of next-generation photovoltaic technologies. In line with the high potential of high-pressure techniques in generating new material structures,39,40,42,76 this work highlights crystallization from the high-energy DAC environment as a simple and straightforward means to discover new phases, even in compositionally simple systems such as PbI2 that has been extensively studied53,54 for almost a century. Whereas the herein described structures of hydrated iodoplumbic acids are so far accessible only at high pressures, and therefore not of immediate importance for the construction of devices, they provide a fundamental advance in the understanding of this important family of materials and a new high-pressure perspective revealing unexpected structures important for the debate on the existence of iodoplumbic acids.
Experimental section
High-pressure experiments
For the high-pressure experiments a liquid solution of PbI2 dissolved in concentrated aqueous hydroiodic acid (HI, 57% by weight) was loaded to a modified Merrill-Bassett diamond anvil cell (DAC). The DAC anvils were supported directly on the steel discs with conical windows (the culet size was 0.8 mm, type 1A diamonds and gasket was made of 0.15 mm thick austenitic steel (type 25-6MO) with the hole diameter 0.45 mm).41 Pressure in the DAC was determined by the ruby fluorescence (R1 ruby line) shift with a photon control spectrometer affording an accuracy of 0.02 GPa.77 Throughout all experiments, the hydrostatic conditions in the DAC were routinely inspected visually by microscopic observations (cf. General microscopy in ESI† for details), by checking the width of the R1 and R2 ruby fluorescence peaks and the widths of SCXRD reflections as well as by searching the background of X-ray images for the presence of diffraction events other than those from the sample crystal. By these means we can be confident that all experiments were conducted in hydrostatic conditions. We attempted high-pressure syntheses and recrystallizations above 3 GPa, however most of the DAC chamber contents froze and its melting required the temperatures approaching the resistance of the steel parts of the DAC and caused difficulties in controlling precisely the temperature and pressure, as required for the growth of single crystals.Diffraction data were collected at 295 K for 1, 3 and 4 and at 320 K for β-PbI2, by using a KM-4 CCD diffractometer with the graphite-monochromated MoKα radiation. The DAC was centered by the gasket-shadow method.78 The CrysAlisCCD and CrysAlisRED programs were used for collecting the data, determination of the UB-matrices, initial data reduction, and Lp correction. Reflection intensities were corrected for the DAC and sample absorption; the gasket shadowing and the reflections of diamond-anvils were eliminated.79 All structures were solved by direct methods, and refined with anisotropic displacement parameters, with programs ShelXS and ShelXL using Olex2 interface.80,81 For re-determination of the structure of 1, the positions of water hydrogen atoms were estimated from the molecular geometry and consistently with the pseudo-hexagonal hydrogen-bonding pattern in the structure. The water molecules were then refined using a rigid-group model, with the isotropic temperature factors of the hydrogen atoms (Uiso) constrained to be 1.5 times the Ueq of the corresponding oxygen atom. Details of structure refinements and crystal data are given in Table S1.† Crystallographic data in CIF format have been deposited with the Cambridge Structural Data Centre, under deposition codes 2071140–2071144.†
High-pressure synthesis of 3 & 4
Single crystals of 3 and 4 were obtained in isochoric conditions: after the polycrystalline mass precipitated, the DAC was heated using a heat gun until all but one grain dissolved. Then the single crystal grew as the DAC was cooled slowly to room temperature, where the pressure was remeasured before and after the X-ray diffraction experiment. The progress and experimental details on growing the single crystals of 3 and 4 are shown in Fig. S1 and S2.†High-pressure synthesis of β-PbI2
Single crystals of β-PbI2 were obtained similarly to those of 3 and 4, however due to the phase transformation at room temperature at 1.2 GPa, the single crystal grown at 2.05 GPa was kept at 320 K and the X-ray diffraction experiment was performed at this temperature.Ball milling experiments
Milling synthesis of 1 : For the ball milling synthesis, 0.461 g (1 mmol) of PbI2 was added to one half of a zirconia (ZrO2) 10 mL jar, followed by the addition of 67 μL (0.5 mmol) of aqueous hydroiodic acid (HI, 57% by weight) and one 3.5 g zirconia ball. The jar was carefully sealed, placed on a MM400 mixer mill and the reaction mixture was milled for 30 minutes at a frequency of 30 Hz. Reaction completion afforded a bright yellow product, which was left to dry in dark for 1 h. The resulting solid product was scraped off the jar walls and subjected to powder X-ray diffraction (PXRD) analysis, revealing the formation of compound 1. Compound 1 can be also isolated when performing the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric reaction by milling 0.230 g (0.5 mmol) of PbI2 with 67 μL (0.5 mmol) of aqueous hydroiodic acid (HI, 57% by weight), respectively. Milling synthesis of2: For the ball-milling synthesis 0.230 g (0.5 mmol) of PbI2 was added to one half of a zirconia (ZrO2) 10 mL jar, followed by the addition of 201 μL (1.5 mmol) of aqueous hydroiodic acid (HI, 57% by weight) and one 3.5 g zirconia ball. The jar was carefully sealed and placed on a MM400 mixer mill and the reaction mixture was milled for 30 minutes at an oscillation rate of 30 Hz. Reaction completion afforded a bright yellow product, which was left to dry in dark overnight. The resulting product scraped off the jar walls was identified as compound 2 by PXRD.
1 stoichiometric reaction by milling 0.230 g (0.5 mmol) of PbI2 with 67 μL (0.5 mmol) of aqueous hydroiodic acid (HI, 57% by weight), respectively. Milling synthesis of2: For the ball-milling synthesis 0.230 g (0.5 mmol) of PbI2 was added to one half of a zirconia (ZrO2) 10 mL jar, followed by the addition of 201 μL (1.5 mmol) of aqueous hydroiodic acid (HI, 57% by weight) and one 3.5 g zirconia ball. The jar was carefully sealed and placed on a MM400 mixer mill and the reaction mixture was milled for 30 minutes at an oscillation rate of 30 Hz. Reaction completion afforded a bright yellow product, which was left to dry in dark overnight. The resulting product scraped off the jar walls was identified as compound 2 by PXRD.
Data availability
Description of pseudosymmetry in 1, experimental procedures and detailed crystallographic data of 3, 4 and β-PbI2, ball-milling experiments, and additional data are located within the ESI.†Author contributions
All high-pressure experiments were performed by S. S., ball milling by A. M. F. and J.-L. D. Research was coordinated and supervised by A. K., T. F., A. M. and G. P. D. All authors discussed the results, contributed to writing the manuscript and commented on it.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We acknowledge the McGill Sustainability Systems Initiative (MSSI) and NSERC Discovery Grant program. S. S. is grateful to the grant POWR.03.02.00-00-I023/17 co-financed by the European Union through the European Social Fund under the Operational Program Knowledge Education Development.References
- J. Euvrard, Y. Yan and D. B. Mitzi, Electrical doping in halide perovskites, Nat. Rev. Mater., 2021, 6, 531–549 CrossRef CAS.
- S. D. Stranks and H. J. Snaith, Metal-halide perovskites for photovoltaic and light-emitting devices, Nat. Nanotechnol., 2015, 10, 391–402 CrossRef CAS PubMed.
- L.-Q. Fan and J.-H. Wu, NH4PbI3, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2007, 63, i189–i189 CrossRef CAS.
- J. Burschka, N. Pellet, S. J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Grätzel, Sequential deposition as a route to high-performance perovskite-sensitized solar cells, Nature, 2013, 499, 316–319 CrossRef CAS PubMed.
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. Il Seok, Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells, Nat. Mater., 2014, 13, 897–903 CrossRef CAS PubMed.
- S. Huang, P. Huang, L. Wang, J. Han, Y. Chen and H. Zhong, Halogenated–Methylammonium Based 3D Halide Perovskites, Adv. Mater., 2019, 31, 1903830 CrossRef CAS PubMed.
- M. Szafrański and A. Katrusiak, Mechanism of pressure-induced phase transitions, amorphization, and absorption-edge shift in photovoltaic methylammonium lead iodide, J. Phys. Chem. Lett., 2016, 7, 3458–3466 CrossRef PubMed.
- M. Szafrański and A. Katrusiak, Photovoltaic hybrid perovskites under pressure, J. Phys. Chem. Lett., 2017, 8, 2496–2506 CrossRef PubMed.
- M. Shirayama, H. Kadowaki, T. Miyadera, T. Sugita, M. Tamakoshi, M. Kato, T. Fujiseki, D. Murata, S. Hara, T. N. Murakami, S. Fujimoto, M. Chikamatsu and H. Fujiwara, Optical Transitions in Hybrid Perovskite Solar Cells: Ellipsometry, Density Functional Theory, and Quantum Efficiency Analyses for CH3NH3PbI3, Phys. Rev. Appl., 2016, 5, 1–25 Search PubMed.
- D. Prochowicz, R. Runjhun, M. M. Tavakoli, P. Yadav, M. Saski, A. Q. Alanazi, D. J. Kubicki, Z. Kaszkur, S. M. Zakeeruddin, J. Lewiński and M. Grätzel, Engineering of perovskite materials based on formamidinium and cesium hybridization for high-efficiency solar cells, Chem. Mater., 2019, 31, 1620–1627 CrossRef CAS.
- Y. Wang, M. I. Dar, L. K. Ono, T. Zhang, M. Kan, Y. Li, L. Zhang, X. Wang, Y. Yang, X. Gao, Y. Qi, M. Grätzel and Y. Zhao, Thermodynamically stabilized β-CsPbI3-based perovskite solar cells with efficiencies >18%, Science, 2019, 365, 591–595 CrossRef CAS PubMed.
- W. Ahmad, J. Khan, G. Niu and J. Tang, Inorganic CsPbI3 Perovskite–Based Solar Cells: A Choice for a Tandem Device, Sol. RRL, 2017, 1, 1–9 Search PubMed.
- T. Baikie, Y. Fang, J. M. Kadro, M. Schreyer, F. Wei, S. G. Mhaisalkar, M. Graetzel and T. J. White, Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications, J. Mater. Chem. A, 2013, 1, 5628–5641 RSC.
- D. Prochowicz, M. Franckevičius, A. M. Cie
![[s with combining umlaut]](https://www.rsc.org/images/entities/char_0073_0308.gif) lak, S. M. Zakeeruddin, M. Grätzel and J. Lewiński, Mechanosynthesis of the hybrid perovskite CH3NH3PbI3: characterization and the corresponding solar cell efficiency, J. Mater. Chem. A, 2015, 3, 20772–20777 RSC.
lak, S. M. Zakeeruddin, M. Grätzel and J. Lewiński, Mechanosynthesis of the hybrid perovskite CH3NH3PbI3: characterization and the corresponding solar cell efficiency, J. Mater. Chem. A, 2015, 3, 20772–20777 RSC. - D. Prochowicz, P. Yadav, M. Saliba, M. Saski, S. M. Zakeeruddin, J. Lewiński and M. Grätzel, Mechanosynthesis of pure phase mixed-cation MAxFA1−xPbI3 hybrid perovskites: photovoltaic performance and electrochemical properties, Sustainable Energy Fuels, 2017, 1, 689–693 RSC.
- W. Huang, J. S. Manser, S. Sadhu, P. V. Kamat and S. Ptasinska, Direct Observation of Reversible Transformation of CH3NH3PbI3 and NH4PbI3 Induced by Polar Gaseous Molecules, J. Phys. Chem. Lett., 2016, 7, 5068–5073 CrossRef CAS PubMed.
- A. Jain, S. P. Ong, G. Hautier, W. Chen, W. D. Richards, S. Dacek, S. Cholia, D. Gunter, D. Skinner, G. Ceder and K. A. Persson, Commentary: The Materials Project: A materials genome approach to accelerating materials innovation, APL Mater., 2013, 1, 011002 CrossRef.
- F. Wang, H. Yu, H. Xu and N. Zhao, HPbI3: A New Precursor Compound for Highly Efficient Solution–Processed Perovskite Solar Cells, Adv. Funct. Mater., 2015, 25, 1120–1126 CrossRef CAS.
- S. Pang, Y. Zhou, Z. Wang, M. Yang, A. R. Krause, Z. Zhou, K. Zhu, N. P. Padture and G. Cui, Transformative Evolution of Organolead Triiodide Perovskite Thin Films from Strong Room-Temperature Solid–Gas Interaction between HPbI3-CH3NH2 Precursor Pair, J. Am. Chem. Soc., 2016, 138, 750–753 CrossRef CAS PubMed.
- Z. Zhou, S. Pang, F. Ji, B. Zhang and G. Cui, The fabrication of formamidinium lead iodide perovskite thin films via organic cation exchange, Chem. Commun., 2016, 52, 3828–3831 RSC.
- M. Long, T. Zhang, Y. Chai, C. F. Ng, T. C. W. Mak, J. Xu and K. Yan, Nonstoichiometric acid–base reaction as reliable synthetic route to highly stable CH3NH3PbI3 perovskite film, Nat. Commun., 2016, 7, 1–11 Search PubMed.
- M. Long, T. Zhang, H. Zhu, G. Li, F. Wang, W. Guo, Y. Chai, W. Chen, Q. Li, K. S. Wong, J. Xu and K. Yan, Textured CH3NH3PbI3 thin film with enhanced stability for high performance perovskite solar cells, Nano Energy, 2017, 33, 485–496 CrossRef CAS.
- F. Ji, S. Pang, L. Zhang, Y. Zong, G. Cui, N. P. Padture and Y. Zhou, Simultaneous Evolution of Uniaxially Oriented Grains and Ultralow-Density Grain-Boundary Network in CH3NH3PbI3 Perovskite Thin Films Mediated by Precursor Phase Metastability, ACS Energy Lett., 2017, 2, 2727–2733 CrossRef CAS.
- Y. Wei, W. Li, S. Xiang, J. Liu, H. Liu, L. Zhu and H. Chen, Precursor effects on methylamine gas-induced CH3NH3PbI3 films for stable carbon-based perovskite solar cells, Sol. Energy, 2018, 174, 139–148 CrossRef CAS.
- X. Ding, H. Chen, Y. Wu, S. Ma, S. Dai, S. Yang and J. Zhu, Triple cation additive NH3+C2H4NH2+C2H4NH3+-induced phase-stable inorganic α-CsPbI3 perovskite films for use in solar cells, J. Mater. Chem. A, 2018, 6, 18258–18266 RSC.
- Z. Liu, L. Qiu, E. J. Juarez-Perez, Z. Hawash, T. Kim, Y. Jiang, Z. Wu, S. R. Raga, L. K. Ono, S. (Frank) Liu and Y. Qi, Gas-solid reaction based over one-micrometer thick stable perovskite films for efficient solar cells and modules, Nat. Commun., 2018, 9, 1–11 CrossRef PubMed.
- T. Zhang, M. I. Dar, G. Li, F. Xu, N. Guo, M. Grätzel and Y. Zhao, Bication lead iodide 2D perovskite component to stabilize inorganic α-CsPbI3 perovskite phase for high-efficiency solar cells, Sci. Adv., 2017, 3, 1–7 Search PubMed.
- S. A. Fateev, E. I. Marchenko, A. A. Petrov, E. A. Goodilin and A. B. Tarasov, New Acidic Precursor and Acetone-Based Solvent for Fast Perovskite Processing via Proton-Exchange Reaction with Methylamine, Molecules, 2020, 25, 1856 CrossRef CAS PubMed.
- W. Ke, I. Spanopoulos, C. C. Stoumpos and M. G. Kanatzidis, Myths and reality of HPbI3 in halide perovskite solar cells, Nat. Commun., 2018, 9, 4785 CrossRef PubMed.
- M. Daub and H. Hillebrecht, On the Demystification of “HPbI3” and the Peculiarities of the Non–innocent Solvents H2O and DMF, Z. Anorg. Allg. Chem., 2018, 644, 1393–1400 CrossRef CAS.
- D. Prochowicz, M. Saski, P. Yadav, M. Grätzel and J. Lewiński, Mechanoperovskites for photovoltaic applications: preparation, characterization, and device fabrication, Acc. Chem. Res., 2019, 52, 3233–3243 CrossRef CAS PubMed.
- Z. Hong, D. Tan, R. A. John, Y. K. E. Tay, Y. K. T. Ho, X. Zhao, T. C. Sum, N. Mathews, F. García and H. S. Soo, Completely solvent-free protocols to access phase-pure, metastable metal halide perovskites and functional photodetectors from the precursor salts, iScience, 2019, 16, 312–325 CrossRef CAS PubMed.
- T. Friščić, C. Mottillo and H. M. Titi, Mechanochemistry for synthesis, Angew. Chem., Int. Ed., 2020, 59, 1018–1029 CrossRef PubMed.
- S. A. Moggach, T. D. Bennett and A. K. Cheetham, The effect of pressure on ZIF–8: increasing pore size with pressure and the formation of a high–pressure phase at 1.47 GPa, Angew. Chem., Int. Ed., 2009, 48, 7087–7089 CrossRef CAS PubMed.
- J. Song, R. Pallach, L. Frentzel-Beyme, P. Kolodzeiski, G. Kieslich, P. Vervoorts, C. L. Hobday and S. Henke, Tuning the High-Pressure Phase Behaviour of Highly Compressible Zeolitic Imidazolate Frameworks: From Discontinuous to Continuous Pore Closure by Linker Substitution, Angew. Chem., Int. Ed., 2022, 61, e202117565 CrossRef CAS PubMed.
- S. Sun, Z. Deng, Y. Wu, F. Wei, F. H. Isikgor, F. Brivio, M. W. Gaultois, J. Ouyang, P. D. Bristowe, A. K. Cheetham and G. Kieslich, Variable temperature and high-pressure crystal chemistry of perovskite formamidinium lead iodide: a single crystal X-ray diffraction and computational study, Chem. Commun., 2017, 53, 7537–7540 RSC.
- L. Zhang, L. Wu, K. Wang and B. Zou, Pressure-Induced Broadband Emission of 2D Organic–Inorganic Hybrid Perovskite (C6H5C2H4NH3)2PbBr4, Adv. Sci., 2019, 6, 2–7 Search PubMed.
- S. Sobczak, P. Ratajczyk and A. Katrusiak, Squeezing Out the Catalysts: A Sustainable Approach to Disulfide Bond Exchange in Aryl Disulfides, ACS Sustainable Chem. Eng., 2021, 9, 7171–7178 CrossRef CAS.
- S. Sobczak and A. Katrusiak, Environment-Controlled Postsynthetic Modifications of Iron Formate Frameworks, Inorg. Chem., 2019, 58, 11773–11781 CrossRef CAS PubMed.
- A. Katrusiak, Lab in a DAC–high-pressure crystal chemistry in a diamond-anvil cell, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2019, 75, 918–926 CrossRef CAS PubMed.
- A. Katrusiak, High-pressure devices, in International Tables for Crystallography Volume H, ed. C. J. Gilmore, J. A. Kaduk and H. Schenk, John Wiley & Sons, Inc., New York, 2018, pp. 156–173 Search PubMed.
- W. Grochala, R. Hoffmann, J. Feng and N. W. Ashcroft, The Chemical Imagination at Work in Very Tight Places, Angew. Chem., Int. Ed., 2007, 46, 3620–3642 CrossRef CAS PubMed.
- M. Szafrański and A. Katrusiak, Phase transitions in layered diguanidinium hexachlorostannate (IV), Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 1026–1035 CrossRef.
- H. Brasseur and L. Pauling, The Crystal Structure of Ammonium Cadmium Chloride, NH4CdCl3, J. Am. Chem. Soc., 1938, 60, 2886–2890 CrossRef CAS.
- D. Bedlivy and K. Mereiter, The structures of potassium lead triiodide dihydrate and ammonium lead triiodide dihydrate, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 782–785 CrossRef.
- C. K. Møller, Crystal Structure and Photoconductivity of Cæsium Plumbohalides, Nature, 1958, 182, 1436–1436 CrossRef.
- H. J. Haupt, F. Huber and H. Preut, Darstellung und Kristallstruktur von Rubidiumtrijodoplumbat(II), Z. Anorg. Allg. Chem., 1974, 408, 209–213 CrossRef CAS.
- D. M. Trots and S. V. Myagkota, High-temperature structural evolution of caesium and rubidium triiodoplumbates, J. Phys. Chem. Solids, 2008, 69, 2520–2526 CrossRef CAS.
- F. Hao, C. C. Stoumpos, Z. Liu, R. P. H. Chang and M. G. Kanatzidis, Controllable perovskite crystallization at a gas–solid interface for hole conductor-free solar cells with steady power conversion efficiency over 10%, J. Am. Chem. Soc., 2014, 136, 16411–16419 CrossRef CAS PubMed.
- S. Alvarez, A cartography of the van der Waals territories, Dalton Trans., 2013, 42, 8617–8636 RSC.
- P. Gilli, L. Pretto, V. Bertolasi and G. Gilli, Predicting Hydrogen-Bond Strengths from Acid–Base Molecular Properties. The pKa Slide Rule: Toward the Solution of a Long-Lasting Problem, Acc. Chem. Res., 2009, 42, 33–44 CrossRef CAS PubMed.
- J. M. Thomas, Topography and topology in solid-state chemistry, Philos. Trans. R. Soc., A, 1974, 277, 251–286 CrossRef CAS.
- P. Terpstra and H. G. Westenbrink, Organic Cation Substitution in Hybrid Perovskite CH3NH3PbI3 with Hydroxylammonium (NH3OH+): A First-Principles Study, Proc. K. Ned. Acad. van Wet., 1926, 29, 431–442 Search PubMed.
- P. A. Beckmann, A review of polytypism in lead iodide, Cryst. Res. Technol., 2010, 45, 455–460 CrossRef CAS.
- C. T. Prewitt and R. T. Downs, Rev. Mineral., 1998, 37, 283 CAS (“high-pressure crystal chemistry”).
- A. Półrolniczak, S. Sobczak and A. Katrusiak, Solid-state associative reactions and the coordination compression mechanism, Inorg. Chem., 2018, 57, 8942–8950 CrossRef PubMed.
- J. F. Ding, P. Cheng, T. T. Ye, W. Xu, H. Zeng, D. Y. Yao, X. M. Pan and J. Zhang, Pressure-Induced Bifurcation in the Photoluminescence of Red Carbon Quantum Dots: Coexistence of Emissions from Surface Groups and Nitrogen-Doped Cores, Appl. Phys. Lett., 2022, 120, 052106 CrossRef CAS.
- P. W. Bridgman, Polymorphic Transitions of 35 Substances to 50,000 Kg/cm3, Proc. Am. Acad. Arts Sci., 1937, 72, 45 CrossRef CAS.
- P. W. Bridgman, Rough Compressions of 177 Substances to 40,000 Kg/cm3, Proc. Am. Acad. Arts Sci., 1948, 76, 71 CrossRef CAS.
- D. Paliwoda, K. F. Dziubek and A. Katrusiak, Imidazole Hidden Polar Phase, Cryst. Growth Des., 2012, 12, 4302–4430 CrossRef CAS.
- H. Tomkowiak, A. Olejniczak and A. Katrusiak, Pressure-Dependent Formation and Decomposition of Thiourea Hydrates, Cryst. Growth Des., 2013, 13, 121–125 CrossRef CAS.
- F. P. A. Fabbiani, D. R. Allan, W. I. F. David, S. A. Moggach, S. Parsons and C. R. Pulham, High-pressure recrystallisation—a route to new polymorphs and solvates, CrystEngComm, 2004, 6, 504–511 RSC.
- J. W. Lee, H. S. Kim and N. G. Park, Lewis Acid–Base Adduct Approach for High Efficiency Perovskite Solar Cells, Acc. Chem. Res., 2016, 49, 311–319 CrossRef CAS PubMed.
- D.-K. Lee, K.-S. Lim, J.-W. Lee and N.-G. Park, Scalable perovskite coating via anti-solvent-free Lewis acid–base adduct engineering for efficient perovskite solar modules, J. Mater. Chem. A, 2021, 9, 3018–3028 RSC.
- A. A. Petrov, N. Pellet, J. Y. Seo, N. A. Belich, D. Y. Kovalev, A. V. Shevelkov, E. A. Goodilin, S. M. Zakeeruddin, A. B. Tarasov and M. Graetzel, New Insight into the Formation of Hybrid Perovskite Nanowires Via Structure Directing Adducts, Chem. Mater., 2017, 29, 587–594 CrossRef CAS.
- A. M. A. Leguy, Y. Hu, M. Campoy-Quiles, M. I. Alonso, O. J. Weber, P. Azarhoosh, M. Van Schilfgaarde, M. T. Weller, T. Bein, J. Nelson, P. Docampo and P. R. F. Barnes, Reversible Hydration of CH3NH3PbI3 in Films, Single Crystals, and Solar Cells, Chem. Mater., 2015, 27, 3397–3407 CrossRef CAS.
- D. Li, S. A. Bretschneider, V. W. Bergmann, I. M. Hermes, J. Mars, A. Klasen, H. Lu, W. Tremel, M. Mezger, H. J. Butt, S. A. L. Weber and R. Berger, Humidity-Induced Grain Boundaries in MAPbI3 Perovskite Films, J. Phys. Chem. C, 2016, 120, 6363–6368 CrossRef CAS.
- A. A. Petrov, S. A. Fateev, V. N. Khrustalev, Y. Li, P. V. Dorovatovskii, Y. V. Zubavichus, E. A. Goodilin and A. B. Tarasov, Formamidinium haloplumbate intermediates: the missing link in a chain of hybrid perovskites crystallization, Chem. Mater., 2020, 32, 7739–7745 CrossRef CAS.
- A. A. Petrov, I. P. Sokolova, N. A. Belich, G. S. Peters, P. V. Dorovatovskii, Y. V. Zubavichus, V. N. Khrustalev, A. V. Petrov, M. Grätzel, E. A. Goodilin and A. B. Tarasov, Crystal Structure of DMF-Intermediate Phases Uncovers the Link Between CH3NH3PbI3 Morphology and Precursor Stoichiometry, J. Phys. Chem. C, 2017, 121, 20739–20743 CrossRef CAS.
- Y. Guo, K. Shoyama, W. Sato, Y. Matsuo, K. Inoue, K. Harano, C. Liu, H. Tanaka and E. Nakamura, Chemical Pathways Connecting Lead(II) Iodide and Perovskite via Polymeric Plumbate(II) Fiber, J. Am. Chem. Soc., 2015, 137, 15907–15914 CrossRef CAS PubMed.
- B. R. Vincent, K. N. Robertson, T. S. Cameron and O. Knop, Alkylammonium lead halides. Part 1. Isolated PbI64− ions in (CH3NH3)4PbI6•2H2O, Can. J. Chem., 1987, 65, 1042–1046 CrossRef CAS.
- J. Cao, X. Jing, J. Yan, C. Hu, R. Chen, J. Yin, J. Li and N. Zheng, Identifying the molecular structures of intermediates for optimizing the fabrication of high-quality perovskite films, J. Am. Chem. Soc., 2016, 138, 9919–9926 CrossRef CAS PubMed.
- H. W. Cremer and D. R. Duncan, CCXLIX.—A study of the polyhalides. Part I. Methods of preparation, J. Chem. Soc., 1931, 1857–1866 RSC.
- J. M. Williams and S. W. Peterson, Example of the [H5O2]+ ion. Neutron diffraction study of tetrachloroauric acid tetrahydrate, J. Am. Chem. Soc., 1969, 91, 776–777 CrossRef CAS.
- J. Cao, X. Jing, J. Yan, C. Hu, R. Chen, J. Yin, J. Li and N. Zheng, Identifying the molecular structures of intermediates for optimizing the fabrication of high-quality perovskite films, J. Am. Chem. Soc., 2016, 138, 9919–9926 CrossRef CAS PubMed.
- X. Dong, A. R. Oganov, A. F. Goncharov, E. Stavrou, S. Lobanov, G. Saleh, G. R. Qian, Q. Zhu, C. Gatti, V. L. Deringer, R. Dronskowski, X. F. Zhou, V. B. Prakapenka, Z. Konôpková, I. A. Popov, A. I. Boldyrev and H. T. Wang, A stable compound of helium and sodium at high pressure, Nat. Chem., 2017, 9, 440–445 CrossRef CAS PubMed.
- G. J. Piermarini, S. Block, J. D. Barnett and R. A. Forman, Calibration of the pressure dependence of the R1 ruby fluorescence line to 195 kbar, J. Appl. Phys., 1975, 46, 2774–2780 CrossRef CAS.
- A. Budzianowski and A. Katrusiak, in High-Pressure Crystallog, Springer Netherlands, Dordrecht, 2004, pp. 101–112 Search PubMed.
- A. Katrusiak, Shadowing and absorption corrections of single-crystal high-pressure data, Z. Kristallogr. – Cryst. Mater., 2004, 219, 461–467 CrossRef CAS.
- G. M. Sheldrick, Crystal Structure Refinement with SHELXL, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, OLEX2: A Complete Structure Solution, Refinement and Analysis Program, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2071140–2071144. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qi01102j |
| ‡ A previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.26434/chemrxiv.14369813.v1). |
| This journal is © the Partner Organisations 2024 |