Optically active poly(diphenylacetylene)s showing solvent-dependent helix inversion accompanied by modulation of helix inversion barriers†
Abilesh Kumar
Ravikumar
a,
Tatsuya
Nishimura
 *b,
Tsuyoshi
Taniguchi‡
*b,
Tsuyoshi
Taniguchi‡
 bc and
Katsuhiro
Maeda
bc and
Katsuhiro
Maeda
 *c
*c
aDivision of Nano Life Science, Graduate School of Frontier Science Initiative, Kanazawa University, Kakuma-machi, Kanazawa 920-1192, Japan
bGraduate School of Natural Science & Technology, Kanazawa University, Kakuma-machi, Kanazawa 920-1192, Japan. E-mail: nishimura@se.kanazawa-u.ac.jp
cWPI Nano Life Science Institute (WPI-NanoLSI), Kanazawa University, Kakuma-machi, Kanazawa 920-1192, Japan. E-mail: maeda@se.kanazawa-u.ac.jp
First published on 3rd July 2024
Abstract
Symmetrically substituted poly(diphenylacetylene)s bearing optically active 2-octyloxycarbonyl groups at the para-positions of the pendant phenyl rings not only show a unique solvent-dependent helix inversion to afford diastereomeric right- and left-handed helical polymers but also significant unprecedented solvent-dependent changes in the helix inversion barrier of the polymer backbone resulting in switching between static or dynamic behavior of the helical polymers at approximately room temperature depending on the solvents used.
Inspired by the structures and functionality of helical constructions in living organisms, the development of optically active covalent and supramolecular helical polymers with controlled helix-sense has garnered considerable attention across the disciplines of chemistry, biology, and materials science.1–6 Owing to their exceptional chiral recognition abilities and chiroptical properties originating from helical chirality, artificial helical polymers hold significant promise in chiral materials and supramolecular architectures7 with diverse applications,2,8,9 including chiral stationary phases for high-performance liquid chromatography,2,10–13 chiral sensors,14,15 chiral catalysts,16–21 and circularly polarized light-emitting materials.22–24 Helix inversion is a prominent feature of dynamic helical polymers, wherein the predominant helix sense of the polymer backbone is inverted in response to external stimuli such as solvents,17,25–27 temperature,28–31 light,32 pressure,33 and metal salts.34 Such a unique dynamic property is expected to facilitate the development of diverse novel functional chiral materials with switchable functions based on the switching of their helical chirality. Indeed, several examples of such have been reported.5,6,8,11,17,19,35–38 Whether a helical polymer exhibits dynamic or static behavior depends on its helix inversion barrier, which is determined by the structure of the polymer.2,8
Poly(diphenylacetylene)s (PDPAs) are a class of π-conjugated helical polymers. Among synthetic helical polymers, PDPAs have attracted particularly significant attention as practical chiral materials owing to their thermal and chemical stabilities, exceptional light resistance, high liquid crystallinity, high gas permeability, and outstanding light storage properties.39–42 However, owing to significant limitations in the methods of polymerizing diphenylacetylenes using the conventional WCl6-based catalyst such as WCl6–Ph4Sn, the synthesis of high-molecular-weight optically active PDPAs with polar functional groups (including ester groups) has proved a considerable challenge.40,43,44 We very recently reported that the MoCl5–PhSnnBu3 system effectively catalyses the polymerization of symmetrically substituted diphenylacetylenes with polar ester groups, allowing the synthesis of high molecular weight PDPAs bearing ester groups.45 In the course of this study, we discovered that a symmetrically substituted PDPA (poly-1R) bearing optically active (R)-2-octyloxycarbonyl groups at the para-position exhibited a unique solvent-dependent helix-sense inversion (Fig. 1). The helix inversion barrier of poly-1R is significantly dependent on the solvent, allowing the nature of poly-1R to be switched between dynamic and static helical polymers at approximately room temperature. To the best of our knowledge, this is the first example of the solvent-dependent helix inversion of PDPAs accompanied by switching between dynamic and static properties of its helical structure in solution.
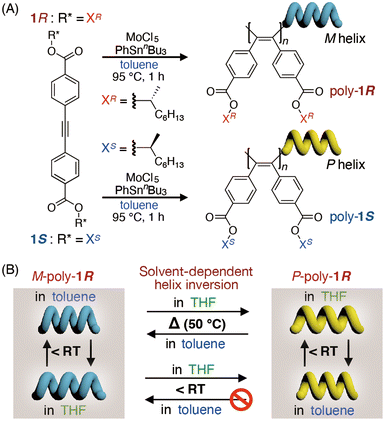 | ||
| Fig. 1 (A) Synthesis of poly-1R and poly-1S. (B) Schematic illustration of solvent-dependent helix inversion accompanied by modulation of its helix inversion barrier in poly-1R (see also Fig. S1, ESI†). | ||
We synthesized a pair of PDPAs bearing optically active (R)- and (S)-2-octyloxycarbonyl groups at the para-positions (poly-1R and poly-1S) via the polymerization of the corresponding diphenylacetylenes (1R and 1S), respectively, in toluene at 95 °C using the MoCl5–PhSnnBu3 catalytic system (Fig. 1A and Table S1, ESI†). Size-exclusion chromatography (SEC) confirmed the very high number-average molar mass (Mn) of the obtained polymers (Mn ≥ 1.4 × 105) with a dispersity (Mw/Mn) of 2.3. Poly-1R and poly-1S are soluble in various solvents including toluene, tetrahydrofuran (THF), chloroform, benzene, chlorobenzene, p-xylene methylcyclohexane (MCH), and ethyl acetate (AcOEt).
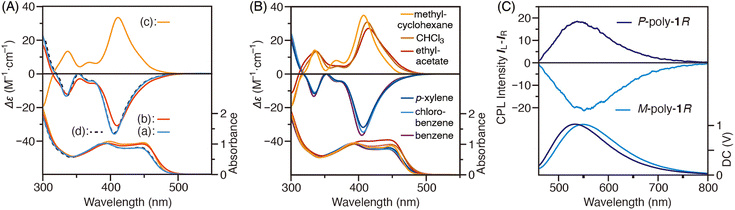
The circular dichroism (CD) and absorption spectra of the as-prepared poly-1R and poly-1S acquired in toluene at 25 °C are shown in Fig. 2A(a) and Fig. S2(a) (ESI†). The CD spectra of the as-prepared poly-1R and poly-1S were mirror-images with an intense negative (Δε1st = −36.0) and positive (Δε1st = +37.0) Cotton effect, respectively, in the absorption region of the polyene backbone, indicating that poly-1R and poly-1S predominantly form a left-handed (M) and right-handed (P) helical structure, respectively, owing to the effect of the optically active 2-octyloxycarbonyl groups in the pendants.46 When dissolved in THF rather than toluene, the as-prepared poly-1R exhibited a slightly less negative Cotton effect (Δε1st = −31.0) at a slightly longer wavelength than that in toluene, which was accompanied by a slight red shift in its absorption spectrum (Fig. 2A(b)). However, the CD and absorption spectra measured in toluene after removing THF (Fig. 2A(d)) were identical to the spectra measured in toluene prior to dissolving in THF (Fig. 2A(a)), suggesting that the as-prepared poly-1R forms slightly different but interconvertible helical conformations in toluene and THF.
Interestingly, a THF solution of poly-1R heated at 50 °C for 12 h showed a positive Cotton effect (Δε1st = +33.3) at 25 °C with essentially no change in absorption spectrum (Fig. 2A(c)), whose CD spectrum is a mirror image of that before heating (Fig. 2A(b)). Similar behavior was also observed for poly-1S (Fig. S2, ESI†). Furthermore, when poly-1R was redissolved in toluene following the removal of THF (Fig. S3(a), ESI†), the CD spectrum was essentially a mirror image of that acquired in toluene before thermal annealing in THF (Fig. 2A(a)). These results indicate that the left-handed helical structure of the thermodynamically stable as-prepared poly-1R (M-poly-1R), which is formed during the polymerization reaction in toluene at 95 °C, is retained as memory in THF at 25 °C; however, thermal annealing in THF causes a solvent-dependent helix-sense inversion to a right-handed helical structure (P-poly-1R), which is thermodynamically more stable in THF, and the inverted helix-sense is also retained as memory in toluene at 25 °C. This solvent-dependent helix-sense inversion of poly-1R was demonstrated to occur reversibly by thermal annealing in THF and toluene, as the P-poly-1R in toluene changed to the M-poly-1R showing the negative Cotton effect upon thermal annealing at 95 °C (Fig. S3(b) and S4, ESI†).
Neither the CD nor absorption spectra of M-poly-1R in toluene, nor those of P-poly-1R in THF showed any concentration dependence (0.1–10 mM) (Fig. S5, ESI†). Furthermore, the hydrodynamic diameters (d) of M-poly-1R in toluene (d = 38.7 nm) and P-poly-1R in THF (d = 42.2 nm) estimated by dynamic light scattering (DLS) were approximately equivalent. These results indicate that the solvent-dependent CD inversion of poly-1R is not a result of aggregation, but rather due to the helix-sense inversion of the polymer backbone in a single polymer chain. Incidentally, limited examples of asymmetrically substituted PDPAs with optically active pendants showing a solvent-dependent helix-sense inversion have been reported so far;37,38 however, the present study is the first example of a solvent-dependent helix inversion in symmetrically substituted PDPAs.
To further investigate the solvent-dependent helix-sense inversion of poly-1R, the CD and absorption spectra of the as-prepared poly-1R were measured after thermal annealing at 50 °C for 12 h in benzene, chlorobenzene, p-xylene, chloroform, MCH and AcOEt (Fig. 2B). Heating in benzene, chlorobenzene and p-xylene produced the same negative Cotton effect as heating in toluene; however, after heating in chloroform, MCH and AcOEt, a positive Cotton effect identical to that in THF was observed (Fig. 2B). Thus, thermal annealing in aromatic and non-aromatic solvents typically reverses the helix-sense of the thermodynamically stable helical structure induced in the main chain of poly-1R.
PDPAs are known to have a relatively high helix inversion barrier and are not susceptible to helix inversion around at room temperature.12,37,38,46 To determine the stability of the helical structure of P-poly-1R obtained by thermal annealing of M-poly-1R in THF, the changes in the CD intensity over time was measured in toluene at 25 °C. After 44 h, the CD intensity decreased by only 6%, indicating that the helical structure of P-poly-1R is strongly retained as memory at 25 °C (Fig. 3A). Moreover, its helical structure was completely maintained even after 8 days at 15 °C, with no reduction in CD intensity (Fig. 3B). Therefore, the circularly polarized luminescence (CPL) spectra of the diastereomeric M-poly-1R and P-poly-1R in toluene, whose CD spectra are essentially mirror images (Fig. S6, ESI†), were measured. Despite both substances having the same chiral substituents in the pendants, the CPL spectra of M-poly-1R and P-poly-1R were essentially mirror images of each other owing to the opposite helix-sense of the polymer backbones (Fig. 2C). The CPL dissymmetry factors of M-poly-1R and P-poly-1R, estimated by glum = 2(IL − IR)/(IL + IR), where IL and IR are the left- and right-handed circularly polarized light, respectively, were 1.4 × 10−3 and 1.2 × 10−3, respectively. The relative quantum yields of M-poly-1R and P-poly-1R in toluene were also determined to be 7.4% and 15%, respectively. Conversely, when the CD intensity changes of M-poly-1R with time was measured in THF at 25 °C, the sign reversed from negative to positive after 20 h. Further, the CD intensity reached a constant value after 43 h, which was identical to that of P-poly-1R in THF (Fig. 3A). Thus, the helix-sense inversion occurs much faster in THF than in toluene, with that in THF reaching completion within two days even at 25 °C. The helix-sense inversion was also observed in THF at 15 °C, which was completed within 8 days (Fig. 3B).
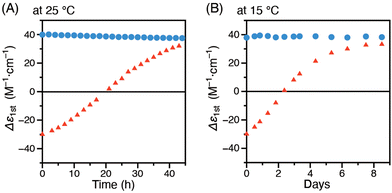 | ||
| Fig. 3 Time-dependent CD intensity (Δε1st) changes of P-poly-1R in toluene (circle) and M-poly-1R in THF (triangle), measured at 25 °C (A) and 15 °C (B). | ||
The changes in CD intensity of M-poly-1R in THF and P-poly-1R in toluene over time were measured at 30, 40, 50, and 60 °C. The pseudo-first-order rate constants were estimated from the reduction in the CD intensity at ca. 400 nm, while the Arrhenius and Eyring plots gave activation parameters for the helix inversion: Ea = 89.0 kJ mol−1 (21.3 kcal mol−1), ΔG‡298 = 86.5 kJ mol−1, ΔH‡ = 86.3 kJ mol−1, and ΔS‡ = −53.1 J (mol K)−1 at T = 298 K (Fig. S7, ESI†). The obtained ΔG‡298 value is similar to the activation energy value (ΔG‡ = 93.7 kJ mol−1) for interconversion of the P- and M-helical conformations of the optically inactive PDPA bearing achiral heptyl ester groups at the para-position in chloroform, which was estimated based on the coalescence temperature and the difference in chemical shifts of the diastereotopic geminal methylene proton 1H NMR signals resulting from the formation of the helical structure.46 In contrast, the activation parameters of the helix inversion in a toluene solution of P-poly-1R, were Ea = 117.6 kJ mol−1 (28.1 kcal mol−1), ΔG‡298 = 115 kJ mol−1, ΔH‡ = 115.1 kJ mol−1 and ΔS‡ = 29.5 J (mol K)−1 (T = 298 K) (Fig. S8, ESI†). Thus, the helix inversion barrier of poly-1R in toluene was significantly greater than that in THF. The reason for this solvent-dependent helicity inversion accompanied with modulation of the helix inversion barrier observed in poly-1R is not currently clear; however, solvation of the chiral alkyl pendants with aromatic solvents may contribute to a conformational change in the chiral pendants, thereby changing the interaction and steric repulsion between the chiral pendants.
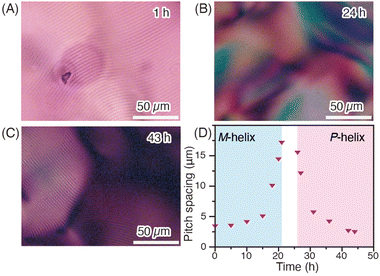
Concentrated solutions of PDPAs are known to exhibit lyotropic liquid crystallinity (LC) owing to the rigid main chain structure of PDPAs, while PDPAs with optically active pendants exhibit cholesteric LC.22,42,47 Polarized microscopy analysis of a concentrated THF solution (28 wt%) of M-poly-1R revealed clear fingerprint textures characteristic of the cholesteric LC (Fig. 4A). Initially, the cholesteric pitch, which was calculated from the spacing of the fringes corresponding to half of the cholesteric pitch, was approximately 3.15 μm, but gradually increased over time. After 24 h, the fingerprint textures disappeared (Fig. 4B), indicating that the concentrated solution transitioned to the nematic LC phase. The observable fingerprint textures appeared after 27 h and the cholesteric pitch gradually decreased with time, reaching a constant value of 2.57 μm after 43 h (Fig. 4C). These results show a strong correlation with the time-dependent CD intensity changes in THF (Fig. 3A), suggesting that the change in the cholesteric pitch arises from the helix-sense inversion of the polymer backbone and that the helix-sense inversion was achieved not only in solution but also in the LC state. Thus, by exploiting the dynamic behavior in THF at approximately room temperature, we observed significant time-dependent changes in the cholesteric pitch (Fig. 4D).
In conclusion, a symmetrically substituted optically active PDPA-bearing chiral 2-octyloxycarbonyl pendants exhibits solvent-dependent helix inversion with a solvent-dependent helix inversion barrier. The structure of the optically active pendant group may play a crucial role in the emergence of the observed switchable chiral properties of this PDPA, and we are currently further investigating this aspect. We believe that this will facilitate the development of novel chiral materials.48
This work was supported by JSPS KAKENHI (JP21K04685, JP21H04691, JP21KK0084, JP24K01466). The authors thank the World Premier International Research Center Initiative (WPI); MEXT, Japan. A. K. R. thanks MEXT fellowship (213418).
Data availability
The data supporting this article have been included in the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- T. Nakano and Y. Okamoto, Chem. Rev., 2001, 101, 4013–4038 CrossRef CAS PubMed
.
- E. Yashima, N. Ousaka, D. Taura, K. Shimomura, T. Ikai and K. Maeda, Chem. Rev., 2016, 116, 13752–13990 CrossRef CAS PubMed
.
- T. Leigh and P. Fernandez-Trillo, Nat. Rev. Chem., 2020, 4, 291–310 CrossRef CAS PubMed
.
- F. García, R. Gómez and L. Sánchez, Chem. Soc. Rev., 2023, 52, 7524–7548 RSC
.
- G. Liu, M. G. Humphrey, C. Zhang and Y. Zhao, Chem. Soc. Rev., 2023, 52, 4443–4487 RSC
.
- M. Lago-Silva, M. Fernández-Míguez, R. Rodríguez, E. Quiñoá and F. Freire, Chem. Soc. Rev., 2024, 53, 793–852 RSC
.
- N. Liu, R.-T. Gao and Z.-Q. Wu, Acc. Chem. Res., 2023, 56, 2954–2967 CrossRef CAS PubMed
.
- E. Yashima and K. Maeda, Bull. Chem. Soc. Jpn., 2021, 94, 2637–2661 CrossRef CAS
.
- N. Liu, L. Zhou and Z. Q. Wu, Acc. Chem. Res., 2021, 54, 3953–3967 CrossRef CAS PubMed
.
- T. Nakano, J. Chromatogr. A, 2001, 906, 205–225 CrossRef CAS PubMed
.
- K. Shimomura, T. Ikai, S. Kanoh, E. Yashima and K. Maeda, Nat. Chem., 2014, 6, 429–434 CrossRef CAS PubMed
.
- K. Maeda, M. Maruta, Y. Sakai, T. Ikai and S. Kanoh, Molecules, 2016, 21, 1487 CrossRef PubMed
.
- J. Shen and Y. Okamoto, Chem. Rev., 2016, 116, 1094–1138 CrossRef CAS PubMed
.
- K. Maeda, D. Hirose, N. Okoshi, K. Shimomura, Y. Wada, T. Ikai, S. Kanoh and E. Yashima, J. Am. Chem. Soc., 2018, 140, 3270–3276 CrossRef CAS PubMed
.
- K. Maeda, D. Hirose, M. Nozaki, Y. Shimizu, T. Mori, K. Yamanaka, K. Ogino, T. Nishimura, T. Taniguchi, M. Moro and E. Yashima, Sci. Adv., 2021, EABG5381 Search PubMed
.
- M. Reggelin, S. Doerr, M. Klussmann, M. Schultz and M. Holbach, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5461–5466 CrossRef CAS PubMed
.
- Y. Nagata, T. Nishikawa and M. Suginome, J. Am. Chem. Soc., 2014, 136, 15901–15904 CrossRef CAS
.
- Y. Nagata, R. Takeda and M. Suginome, ACS Cent. Sci., 2019, 5, 1235–1240 CrossRef CAS PubMed
.
-
M. Suginome, Molecular Technology, 2019, pp. 77–94 DOI:10.1002/9783527823987.vol4_c4
.
- T. Ikai, M. Ando, M. Ito, R. Ishidate, N. Suzuki, K. Maeda and E. Yashima, J. Am. Chem. Soc., 2021, 143, 12725–12735 CrossRef CAS PubMed
.
- Z.-Q. Wu, X. Song, Y.-X. Li, L. Zhou, Y.-Y. Zhu, Z. Chen and N. Liu, Nat. Commun., 2023, 14, 566 CrossRef CAS PubMed
.
- K. Akagi, Bull. Chem. Soc. Jpn., 2019, 92, 1509–1655 CrossRef CAS
.
- S. Wang, D. Hu, X. Guan, S. Cai, G. Shi, Z. Shuai, J. Zhang, Q. Peng and X. Wan, Angew. Chem., Int. Ed., 2021, 60, 21918–21926 CrossRef CAS PubMed
.
- S. Sona, D. Hirose, Y. Kurihara and K. Maeda, J. Mater. Chem. C, 2023, 11, 1271–1277 RSC
.
- K. Okoshi, S.-i Sakurai, S. Ohsawa, J. Kumaki and E. Yashima, Angew. Chem., Int. Ed., 2006, 45, 8173–8176 CrossRef CAS PubMed
.
- S.-i Sakurai, K. Okoshi, J. Kumaki and E. Yashima, J. Am. Chem. Soc., 2006, 128, 5650–5651 CrossRef CAS
.
- T. Yamada, Y. Nagata and M. Suginome, Chem. Commun., 2010, 46, 4914–4916 RSC
.
- K. Maeda and Y. Okamoto, Macromolecules, 1998, 31, 5164–5166 CrossRef CAS PubMed
.
- M. Fujiki, J. Am. Chem. Soc., 2000, 122, 3336–3343 CrossRef CAS
.
- K. Maeda, K. Shimomura, T. Ikai, S. Kanoh and E. Yashima, Macromolecules, 2017, 50, 7801–7806 CrossRef CAS
.
- X. Guan, S. Wang, G. Shi, J. Zhang and X. Wan, Macromolecules, 2021, 54, 4592–4600 CrossRef CAS
.
- G. Maxein and R. Zentel, Macromolecules, 1995, 28, 8438–8440 CrossRef CAS
.
- Y. Nagata, R. Takeda and M. Suginome, RSC Adv., 2016, 6, 109726–109729 RSC
.
- F. Freire, J. M. Seco, E. Quiñoá and R. Riguera, Angew. Chem., Int. Ed., 2011, 50, 11692–11696 CrossRef CAS PubMed
.
- Y. Nagata, T. Nishikawa and M. Suginome, Chem. Commun., 2014, 50, 9951–9953 RSC
.
- D. Hirose, A. Isobe, E. Quiñoá, F. Freire and K. Maeda, J. Am. Chem. Soc., 2019, 141, 8592–8598 CrossRef CAS PubMed
.
- D. Hirose, M. Nozaki, M. Maruta and K. Maeda, Chirality, 2022, 34, 597–608 CrossRef CAS PubMed
.
- J. J. Tarrío, R. Rodríguez, B. Fernández, E. Quiñoá and F. Freire, Angew. Chem., Int. Ed., 2022, 61, e202115070 CrossRef PubMed
.
- T. Aoki, T. Kaneko and M. Teraguchi, Polymer, 2006, 47, 4867–4892 CrossRef CAS
.
- T. Masuda, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 165–180 CrossRef CAS
.
- J. Liu, J. W. Lam and B. Z. Tang, Chem. Rev., 2009, 109, 5799–5867 CrossRef CAS PubMed
.
- Y.-J. Jin and G. Kwak, Polym. Rev., 2017, 57, 175–199 CrossRef CAS
.
- T. Masuda, H. Kawai, T. Ohtori and T. Higashimura, Polym. J., 1979, 11, 813–818 CrossRef CAS
.
- M. Miyairi, T. Taniguchi, T. Nishimura and K. Maeda, Angew. Chem., Int. Ed., 2020, 59, 14772–14780 CrossRef CAS PubMed
.
- M. Miyairi, T. Taniguchi, T. Nishimura and K. Maeda, Angew. Chem., Int. Ed., 2023, 62, e202302332 CrossRef CAS PubMed
.
- K. Maeda, M. Nozaki, K. Hashimoto, K. Shimomura, D. Hirose, T. Nishimura, G. Watanabe and E. Yashima, J. Am. Chem. Soc., 2020, 142, 7668–7682 CrossRef CAS PubMed
.
- B. A. San Jose, S. Matsushita and K. Akagi, J. Am. Chem. Soc., 2012, 134, 19795–19807 CrossRef CAS PubMed
.
- S. Mishra, A. K. Mondal, E. Z. B. Smolinsky, R. Naaman, K. Maeda, T. Nishimura, T. Taniguchi, T. Yoshida, K. Takayama and E. Yashima, Angew. Chem., Int. Ed., 2020, 59, 14671–14676 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, characterizations of polymers, and supporting data (PDF). See DOI: https://doi.org/10.1039/d4cc02656j |
| ‡ Present address: Interdisciplinary Research Center for Catalytic Chemistry, National Institute of Advanced Industrial Science and Technology (AIST), 1-1-1 Higashi, Tsukuba, Ibaraki 305-8565, Japan. |
| This journal is © The Royal Society of Chemistry 2024 |