Open Access Article
Open Access ArticleHighly substituted benzo[b]furan synthesis through substituent migration†
Akihiro
Kobayashi
ab,
Shinya
Tabata
a and
Suguru
Yoshida
 *a
*a
aDepartment of Biological Science and Technology, Faculty of Advanced Engineering, Tokyo University of Science, 6-3-1 Niijuku, Katsushika-ku, Tokyo 125-8585, Japan. E-mail: s-yoshida@rs.tus.ac.jp
bLaboratory of Chemical Bioscience, Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University (TMDU), 2-3-10 Kanda-Surugadai, Chiyoda-ku, Tokyo 101-0062, Japan
First published on 28th March 2024
Abstract
An unusual benzofuran synthesis from 2,6-disubstituted phenols and alkynyl sulfoxides is disclosed. Various highly substituted benzofurans were synthesized via the charge-accelerated [3,3]-sigmatropic rearrangement and subsequent substituent migration. Multiaryl-substituted benzofurans and fully substituted benzofurans were prepared on the basis of the unique reaction mechanism.
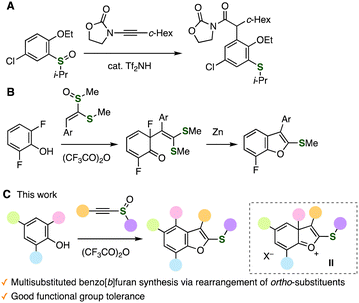
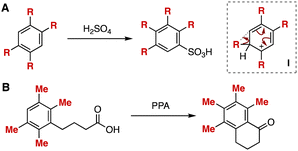
The migration of substituents on aromatic rings has gained attention from organic chemists due to unique mechanisms as well as useful methods to prepare highly substituted arenes. In particular, the migration of alkyl groups on multisubstituted benzenes is known as the Jacobsen rearrangement and enables the synthesis of congested benzenes.1 For example, 2,3,4,5-tetraalkylbenzenesulfonic acids were obtained from 1,2,4,5-tetraalkylbenzenes by sulfonylation in concentrated sulfonic acid through the rearrangement in carbocation intermediates I (Fig. 1A).1c Also, tetramethyl-substituted α-tetralone synthesis was achieved from 4-(2,3,5,6-tetramethylphenyl)butyric acid via the migration of methyl groups (Fig. 1B).1d Despite continuous studies for the rearrangement of cationic intermediates, it is still challenging to synthesize highly functionalized arenes through substituent migration in cationic intermediates.2 Herein, we disclose an unexpected reaction through charge-accelerated [3,3]-sigmatropic rearrangement of sulfonium intermediates followed by substituent migration.
 | ||
| Fig. 1 (A) Alkyl migration in sulfonylation. (B) Methyl migration in cyclization. PPA = polyphosphoric acid. | ||
Recently, remarkable achievements in diverse transformations of sulfoxides through charge-accelerated [3,3]-sigmatropic rearrangement have enabled us to synthesize a wide variety of functionalized arenes owing to the intriguing reactivities of sulfonium intermediates.3,4 A few examples of the [3,3]-sigmatropic rearrangement followed by migration of substituents realized the preparation of multisubstituted arenes in unusual manners. For instance, Maulide and coworkers developed a meta-selective rearrangement using o-substituted phenyl sulfoxides and ynamides catalyzed by Tf2NH through charge-accelerated [3,3]-sigmatropic rearrangement and subsequent alkyl migration (Fig. 2A).5a On the other hand, benzofuran synthesis via dearomatized C–C formation of 2,6-difluorophenols was accomplished through the extended Pummerer reaction of sulfoxides facilitated by TFAA, where deprotonation took place to provide dearomatized intermediates without migration of an alkenyl or a fluoro group (Fig. 2B).6 Considering these contrasting products from similar sulfonium intermediates, it is not easy to promote the rearrangement of ortho-substituent on aromatic rings. In contrast, we found a new method to prepare highly substituted benzo[b]furans from 2,6-substituted phenols with alkynyl sulfoxides in the presence of trifluoroacetic acid anhydride (TFAA) through substituted migration of cationic intermediate II (Fig. 1C).
Unusual substituent migration was found in the benzofuran synthesis from 2-substituted phenols with alkynyl sulfoxide 2a facilitated by TFAA (Fig. 3A).7 The reaction using 2-trifluoromethylphenol (1a) took place smoothly to afford 7-trifluoromethyl-substituted benzofuran 3avia C–C formation at the sterically unhindered site (Fig. 3A, upper). To our surprise, treatment of o-cresol (1b) with alkynyl sulfoxide 2a in the presence of TFAA provided not only 7-methyl-substituted benzofuran 3b but also 4-methyl-substituted benzofuran 4b as an inseparable mixture (Fig. 3A, middle). When 2,5-dimethylphenol (1c) was treated with 2a and TFAA, 4,6-dimethyl-substituted benzofuran 4c was obtained as a major product along with 4,7-dimethylbenzofuran 3c (Fig. 3A, lower). These results obviously showed that unusual substituent migration of cationic intermediates would be involved in the formation of benzofurans 4b and 4c although normal C–C formation also occurred to furnish benzofurans 3b and 3c by avoiding the steric repulsion at the ortho-substituents.
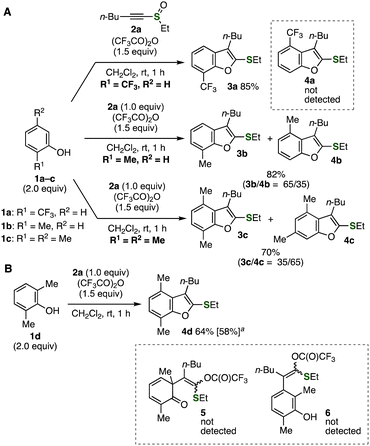 | ||
Fig. 3 (A) Benzofuran synthesis using phenols 1a–c. (B) Benzofuran synthesis using 2,6-dimethylphenol (1d). a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) The reaction was conducted on a 1 mmol scale. The reaction was conducted on a 1 mmol scale. | ||
We succeeded in the selective synthesis of 4,7-dimethyl-substituted benzofuran 4d (= 3c) in good yield by the reaction of 2,6-dimethylphenol (1d) (Fig. 3B). In this reaction, C–C formation at the methyl-substituted carbon of phenol 1d with alkynyl sulfoxide 2a, migration of the methyl group to the neighboring site, and deprotonation would lead to the efficient formation of benzofuran 4d. In contrast to the previous reports on the charge-accelerated [3,3]-sigmatropic rearrangement through aryloxysulfonium intermediates, dearomatized product 5 and 2,3,6-trisubstituted phenol 6 were not observed in the 1H NMR analysis of the crude product after aqueous workup. Synthesis of benzofuran 4d was also accomplished with good yield when the reaction was conducted on a 1 mmol scale.
A wide variety of tetra- or pentasubstituted benzofurans 4e–4l were synthesized from 2,6-disubstituted phenols 1 (Fig. 4). Indeed, pentasubstituted benzofuran 4e was successfully prepared in high yield without damaging the ester moiety. Efficient synthesis of diaryl ketones 4f and 4g was achieved using hydroxy-substituted diaryl ketones keeping ketone, methoxy, and bromo moieties unreacted. It is worth noting that treatment of 4-(pinacolatoboryl)-2,6-dimethylphenol (1h) with alkynyl sulfoxide 2a and TFAA afforded pentasubstituted benzofuran 4h having a transformable boryl group. A range of functional groups were migrated to the neighboring position. For example, we prepared 4,7-diphenylbenzofuran 4i from 2,6-diphenylphenol (1i) and alkynyl sulfoxide 2a in moderate yield through the migration of the phenyl group. Also, 4,7-dibromo-substituted benzofuran 4j was synthesized from 2,6-dibromophenol (1j) via bromo migration. When using 2-ethyl-6-methylphenol (1k), an inseparable mixture of 4k and 4k′ was obtained through the migration of an ethyl or a methyl group, in which the formation of benzofuran 4kvia the migration of the methyl group was slightly favored.1d In addition, we succeeded in the selective synthesis of pentasubstituted benzofuran 4l bearing ethylthio, butyl, methyl, methoxycarbonyl, and bromo groups through methyl migration.
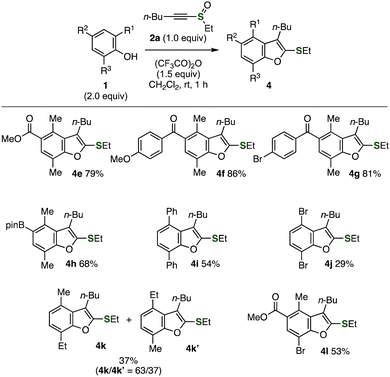 | ||
| Fig. 4 Synthesis of benzofurans using various 2,6-disubstituted phenols. See the ESI† for details. | ||
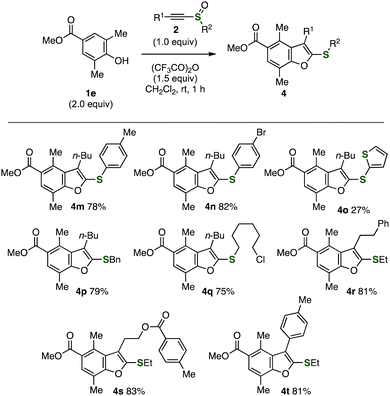
The good functional group tolerance allowed us to synthesize diverse benzofurans 4 possessing a variety of functional groups from various alkynyl sulfoxides 2 (Fig. 5). For instance, we succeeded in the synthesis of aryl benzofuranyl sulfides 4m–o bearing tolyl, 4-bromophenyl, and 2-thienyl groups. Efficient syntheses of benzyl sulfide 4p and 6-chlorohexyl sulfide 4q were accomplished in high yields through methyl migration without damaging benzyl and chloro groups. When using alkynyl sulfoxide having phenethyl, 2-(4-methylbenzoyloxy)ethyl, or 4-tolyl group, pentasubstituted benzofurans 4r–t were prepared in good yields. Due to the good accessibility of divergent alkynyl sulfoxides from terminal alkynes and sulfur surrogates,8 highly functionalized benzofurans will be synthesized from a variety of alkynyl sulfoxides with a broad range of 2,6-disubstituted phenols via the substituent migration.
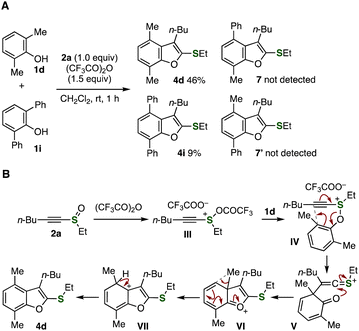
To gain insight into the reaction mechanism, we then performed a competition experiment using phenols 1d and 1i using alkynyl sulfoxide 2a (Fig. 6A). Indeed, treatment of an equimolar mixture of 2,6-dimethylphenol (1d) and 2,6-diphenylphenol (1i) with alkynyl sulfoxide 2a in the presence of TFAA provided dimethyl-substituted benzofuran 4d as a major product along with diphenyl-substituted benzofuran 4i as a minor product. This result indicates that phenol 1d having electron-donating alkyl groups showed higher reactivity than phenol 1i having electron-withdrawing aryl groups. In addition, benzofurans 7 and 7′ bearing both methyl and phenyl groups were not observed, clearly showing that the intramolecular migration step should be involved in the mechanism.
We propose a reaction mechanism through [3,3]-sigmatropic rearrangement followed by substituent migration (Fig. 6B). According to our previous report, electrophilic activation of alkynyl sulfoxide 2a with TFAA first takes place to afford sulfonium (or sulfuran) intermediate III. Then, a substitution reaction of sulfonium intermediate III with phenol 1d and charge-accelerated [3,3]-sigmatropic rearrangement of alkynyl sulfonium intermediate IV furnish dearomatized intermediate V.9 The aromatized structure formed by ring closure, subsequent methyl migration, and deprotonation will provide 4,7-dimethyl-substituted benzofuran 4d. In the ring-closing step, the C–O formation would be facilitated owing to the highly electron-deficient nature of thionium intermediate V, which prevents the migration of the alkynyl sulfoxide unit, leading to clear differences from the previous reports by Maulide or Yorimitsu.5,6
We showcased the synthetic utility of unusual migrative benzofuran formation by sequential cross-coupling reactions (Fig. 7A).10 First, the selective synthesis of benzofuran 9 having an ethylthio group was accomplished from 2-bromo-6-phenylphenol (1m) and alkynyl sulfoxide 2avia the migration of a phenyl group followed by Suzuki–Miyaura cross-coupling with arylboronic acid 8 at the bromo group efficiently without damaging the sulfide moiety.11 Then, we succeeded in the further arylation of sulfide 9 with 4-tolylmagnesium bromide (10) in the presence of a catalytic amount of IPrNiCl2(PPh3).12 Since multisubstituted benzofuran 11 was synthesized from phenol 1m, alkynyl sulfoxide 2a, arylboronic acid 8, and arylmagnesium bromide 10 in a modular synthetic manner, diverse highly substituted benzofurans would be prepared from easily available starting materials by the migrative benzofuran synthesis using 2,6-disubstituted phenols, organoborons, and Grignard reagents.
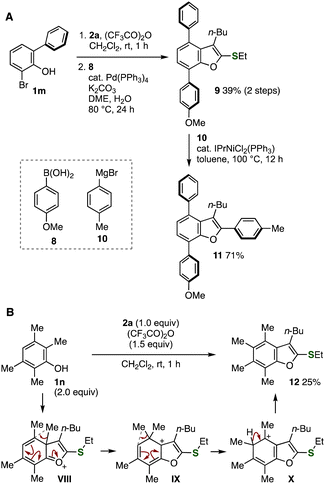 | ||
| Fig. 7 (A) Synthesis of benzofuran 11. (B) Synthesis of benzofuran 12 from 2,3,5,6-tetramethylphenol (1n). | ||
Inspired by the Jacobsen rearrangement in sulfonylation of 2,3,5,6-tetramethylbenzene,1c we accomplished the synthesis of fully substituted benzofuran 12 (Fig. 7B). Indeed, benzofuran 12 was successfully prepared by the treatment of 2,3,5,6-tetramethylphenol (1n) with alkynyl sulfoxide 2a in the presence of TFAA. This result clearly demonstrates that the formation of fully substituted benzofuran 12 would be achieved via sequential migrations of methyl groups via intermediates VIII and IX followed by deprotonation. The unusual transformations via substituent migration of cationic intermediates would serve in synthesizing highly substituted arenes difficult to prepare by conventional methods.
In summary, we found an unusual substituent migration in the charge-accelerated [3,3]-sigmatropic rearrangement using 2,6-disubstituted phenols and alkynyl sulfoxides. A wide range of highly substituted benzofurans were successfully prepared through the [3,3]-sigmatropic rearrangement followed by the substituent migration. Since diverse benzofurans have served as significant bioactive compounds,13 newly accessible multisubstituted benzofurans will be of great importance in pharmaceutical sciences and agrochemistry. Applications to the development of bioactive benzofurans, the synthesis of various heteroaromatics through similar reaction mechanisms, and theoretical studies with DFT calculations are ongoing in our laboratory.
The authors thank Central Glass Co., Ltd. for providing Tf2O. This work was supported by JSPS KAKENHI Grant Number JP22H02086 (S. Y.); The Uehara Memorial Foundation (S. Y.); Tokuyama Science Foundation (S. Y.); The Ube Foundation (S. Y.); Inamori Research Grants (S. Y.); and JST SPRING Grant Number JPMJSP2120 (A. K.).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) O. Jacobsen, Ber., 1886, 19, 1209 CrossRef; (b) H. Suzuki, Bull. Chem. Soc. Jpn., 1963, 36, 1642 CrossRef CAS; (c) R. T. Arnold and R. A. Barnes, J. Am. Chem. Soc., 1944, 66, 960 CrossRef CAS; (d) W. L. Mosby, J. Am. Chem. Soc., 1952, 74, 2564 CrossRef CAS; (e) E. N. Marvell and D. Webb, J. Org. Chem., 1962, 27, 4408 CrossRef CAS.
- For recent examples, see: (a) J. Wang, H.-T. Zhu, Y.-F. Qiu, Y. Niu, S. Chen, Y.-X. Li, X.-Y. Liu and Y.-M. Liang, Org. Lett., 2015, 17, 3186 CrossRef CAS PubMed; (b) S. Maiti and P. Mal, Org. Lett., 2017, 19, 2454 CrossRef CAS PubMed; (c) Y. Ishida, I. Nakamura and M. Terada, J. Am. Chem. Soc., 2018, 140, 8629 CrossRef CAS PubMed; (d) J. Lee, B. Kang, D. Kim, J. Lee and S. Chang, J. Am. Chem. Soc., 2021, 143, 18406 CrossRef CAS PubMed.
- (a) H. Yorimitsu, Chem. Rec., 2017, 17, 1156 CrossRef CAS PubMed; (b) D. Kaiser, I. Klose, R. Oost, J. Neuhaus and N. Maulide, Chem. Rev., 2019, 119, 8701 CrossRef CAS PubMed; (c) Z. He, A. P. Pulis, G. J. P. Perry and D. J. Procter, Phosphorus, Sulfur Silicon Relat. Elem., 2019, 194, 669 CrossRef CAS; (d) W. W. Chen, A. B. Cuenca and A. Shafir, Angew. Chem., Int. Ed., 2020, 59, 16294 CrossRef CAS PubMed; (e) K. Higuchi and M. Tayu, Heterocycles, 2021, 102, 783 CrossRef CAS; (f) Y. Liang and B. Peng, Acc. Chem. Res., 2022, 55, 2103 CrossRef CAS PubMed.
- (a) H. J. Shrives, J. A. Fernández-Salas, C. Hedtke, A. P. Pulis and D. J. Procter, Nat. Commun., 2017, 8, 14801 CrossRef PubMed; (b) Z. He, H. J. Shrives, J. A. Fernández-Salas, A. Abengózar, J. Neufeld, K. Yang, A. P. Pulis and D. J. Procter, Angew. Chem., Int. Ed., 2018, 57, 5759 CrossRef CAS PubMed; (c) K. Yang, A. P. Pulis, G. J. P. Perry and D. J. Procter, Org. Lett., 2018, 23, 7498 CrossRef PubMed; (d) M. Tayu, A. Rahmanudin, G. J. P. Perry, R. U. Khan, D. J. Tate, R. Marcial-Hernandez, Y. Shen, I. Dierking, Y. Janpatompong, S. Aphichatpanichakul, A. Zamhuri, I. Victoria-Yrezabal, M. L. Turner and D. J. Procter, Chem. Sci., 2022, 13, 421 RSC; (e) R. Bisht, M. V. Popescu, Z. He, A. M. Ibrahim, G. E. M. Crisenza, R. S. Paton and D. J. Procter, Angew. Chem., Int. Ed., 2023, 62, e202302418 CrossRef CAS PubMed.
- (a) B. Maryasin, D. Kaldre, R. Galaverna, I. Klose, S. Ruider, M. Drescher, H. Kählig, L. González, M. N. Eberlin, I. D. Jurberg and N. Maulide, Chem. Sci., 2018, 9, 4124 RSC; (b) R. Wakabayashi, M. Fukazawa, T. Kurogi and H. Yorimitsu, Bull. Chem. Soc. Jpn., 2024, 97, uoae002 CrossRef.
- K. Okamoto, M. Hori, T. Yanagi, K. Murakami, K. Nogi and H. Yorimitsu, Angew. Chem., Int. Ed., 2018, 57, 14230 CrossRef CAS PubMed.
- A. Kobayashi, T. Matsuzawa, T. Hosoya and S. Yoshida, RSC Adv., 2023, 13, 839 RSC.
- (a) K. Kanemoto, S. Yoshida and T. Hosoya, Org. Lett., 2019, 21, 3172 CrossRef CAS PubMed; (b) E. Godin, J. Santandrea, A. Caron and S. K. Collins, Org. Lett., 2020, 22, 5905 CrossRef CAS PubMed; (c) A. Kobayashi, T. Matsuzawa, T. Hosoya and S. Yoshida, Chem. Commun., 2020, 56, 5429 RSC; (d) Q. Liu, X.-B. Li, M. Jiang, Z.-J. Liu and J.-T. Liu, Tetrahedron, 2021, 83, 131994 CrossRef CAS.
- V. S. P. R. Lingam, R. Vinodkumar, K. Mukkanti, A. Thomas and B. Gopalan, Tetrahedron Lett., 2008, 49, 4260 CrossRef.
- (a) P. Dobrounig, M. Trobe and R. Breinbauer, Monatsh. Chem., 2017, 148, 3 CrossRef CAS PubMed; (b) S. E. Hooshmand, B. Heidari, R. Sedghi and R. S. Varma, Green Chem., 2019, 21, 381 RSC.
- M. Onoda, Y. Koyanagi, H. Saito, M. Bhanuchandra, Y. Matano and H. Yorimitsu, Asian J. Org. Chem., 2017, 6, 257 CrossRef CAS.
- K. Murakami, H. Yorimitsu and A. Osuka, Angew. Chem., Int. Ed., 2014, 53, 7510 CrossRef CAS PubMed.
- Y.-h Miao, Y.-h Hu, J. Yang, T. Liu, J. Sun and X.-j Wang, RSC Adv., 2019, 9, 27510 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization of new compounds including NMR spectra. See DOI: https://doi.org/10.1039/d4cc01192a |
| This journal is © The Royal Society of Chemistry 2024 |