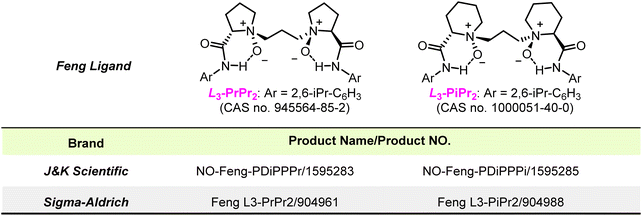
Feng chiral N,N′-dioxide ligands: uniqueness and impacts
Dian-Feng
Chen
 and
Liu-Zhu
Gong
and
Liu-Zhu
Gong
 *
*
Hefei National Research Center for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, Hefei 230026, China. E-mail: gonglz@ustc.edu.cn
First published on 15th June 2023
Abstract
From the historical perspective, all widely accepted privileged chiral ligands tune the catalytic activity of the metal complexes that they form and impart stereoselectivity to asymmetric reactions exclusively relying on the coordination with phosphorus, nitrogen or hetero-atom hybrids. Nowadays, works with N,N’-dioxide amides, i.e., Feng ligands, break with this tradition and have shown that oxygen-coordinated ligands can also be privileged, allowing for the proliferation of chiral metal catalysts and highly enantioselective transformations.
In nature, mirror-image molecules (enantiomers) show dramatically distinct activities, and thus a major focus of research worldwide involves having reactions yield products in an enantioselective manner.1 Enzymes, either naturally occurring or those evolved in the laboratory,2 provide a fascinating approach to asymmetric synthesis, but all too often display insufficient stability and a relatively narrow substrate scope. Other endeavors have been directed toward artificial chiral metal catalysis3 and organocatalysis,4,5 for which the 2001 and 2021 Nobel prizes in Chemistry were awarded, specifically for the development of useful tools and reactions. Arguably, metal complexes with chiral organic ancillaries (namely ligands) are among the most versatile catalysts for constructing stereocenters from prochiral or racemic substrates. Although numerous breakthroughs have been made, identification of the right metal catalyst for complicated substrates or an unprecedented transformation is never an easy task. As such, rational ligand design stands as an essential step, perhaps the essential step, in chiral organometallic chemistry—and the ever-increasing needs for generalizing and predicting ligand–metal-substrate interactions set an ambitious goal: achieving truly privileged ligands.6
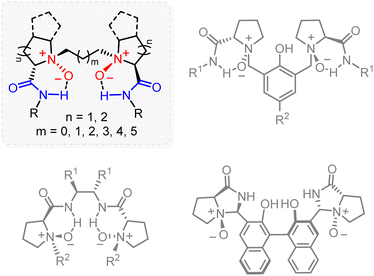
Thus far, all widely accepted privileged chiral ligands tune the activity of the metal complexes that they form and impart stereoselectivity to asymmetric reactions by exclusively relying on coordination with phosphorus or nitrogen atoms.6,7,8,9 Over the past two decades, the Feng group has established a library of chiral N,N’-dioxide amides (Fig. 1), which have emerged as a class of unique and privileged ligands and have enabled the development of major mechanistically unrelated reactions with exceptional levels of regio-, chemo-, diastereo-, and enantioselectivity.10,11,12–15 Such chiral N,N’-dioxide amides are, naturally, known as Feng ligands.16 Since a few comprehensive reviews have nicely recalled the discovery and summarized the applications of Feng ligands, we provide in this highlight a brief discussion focused on the uniqueness and impacts of this class of ligands.
1. Uniqueness of Feng ligands
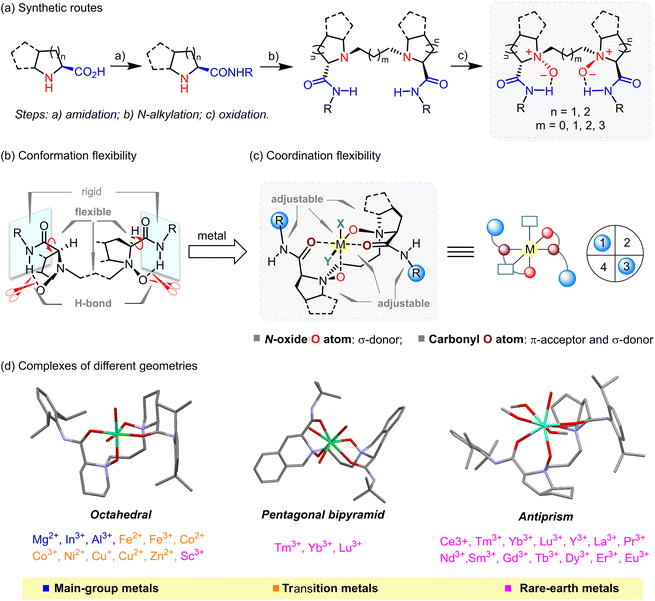
In chiral ligand design, deployment of symmetry17,18,19 and scaffold rigidity6,9,20 can generally minimize the potential number of isomers produced of any ligand–metal-substrate complex by minimizing the number of potential reaction pathways, thus imparting excellent levels of stereocontrol. Adhering to such approaches has proven to be successful and has aided in the discovery of several privileged ligands, including BINAP, bis(oxazolines), salen- and SPINOL-based ligands.6 Indeed, the high performance of a Feng ligand is intimately related to its C2 symmetry. On the other hand, rather than being rigid, Feng ligands are generally flexible, though the first reported Feng ligand employed a somewhat rigid Ti4+ complex.21Conformational flexibility
When starting from commercially available and inexpensive chiral amino acids, the synthesis of a Feng ligand can be completed in three high-yielding transformations: amidation, N-alkylation, and oxidation (Scheme 1a). Notably, inclusion of the amide N–H bond can significantly improve the stability of polar N-oxides and anchor the chirality at nitrogen stereocenters through intramolecular hydrogen bonding.22 As such, Feng ligands tolerate air and moisture such that no special handling is needed for their storage. More importantly, the ease of rotation about the C(sp3)–C(sp3) bonds and the amide C(sp3)–C(sp2) bonds contribute to the overall flexibility of Feng ligands (Scheme 1b).Coordination flexibility
The typical Feng ligand is a neutral, polar, and tetradentate O-donor ligand with two oxygen atoms from amine oxides and the other two from amides. When the Feng ligand interacts with a metal ion, an exchange process occurs, in which oxygen atoms of N-oxides and amides bind to the metal center, and the amide N–H bonds become free to form hydrogen bonds with appropriate substrates (Scheme 1c). Upon such coordination, the alkyl linkage and the amide motifs would shield oppositely related regions (the upper left and lower right quadrants in Scheme 1c) to create a chiral pocket, in which the chelated metal center interacts with substrates in an enantioselective manner.11 Note that N-oxides can function as donors of σ-electron density, while amides act as σ-donors and π-acceptors,23 thus accommodating distinct metal ions having either nearly filled or empty d orbitals of correct symmetry.Such unique conformational and coordination flexibility makes the Feng ligand design truly versatile. In fact, Feng ligands readily chelate with more than 20 metal ions, ranging from main-group and transition metals to rare-earth metals. Specifically, octahedral complexes are formed with metal ions of small ionic radius, including main-group and transition metals, as well as Sc3+. Also note that the chemical structure of the Feng ligand design significantly influences the manner of its coordination. Specifically, a distorted trigonal bipyramidal Co2+ complex with five coordinating ligands was recently reported by Feng and coworkers.24 For larger metal ions, e.g., the majority of rare-earth metal salts, Feng ligands tend to chelate and afford complexes with increased coordination number and distorted geometries (Scheme 1d).14 And as a practical matter, these metallic complexes have proven to be enantioselective over a wide range of mechanistically unrelated reactions, which firmly underpins the “privilege” of the ligands.
2. Impacts of the Feng ligand
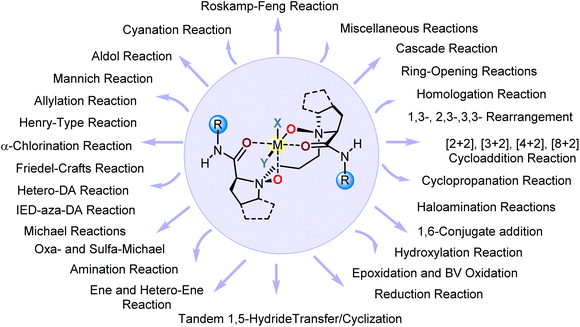
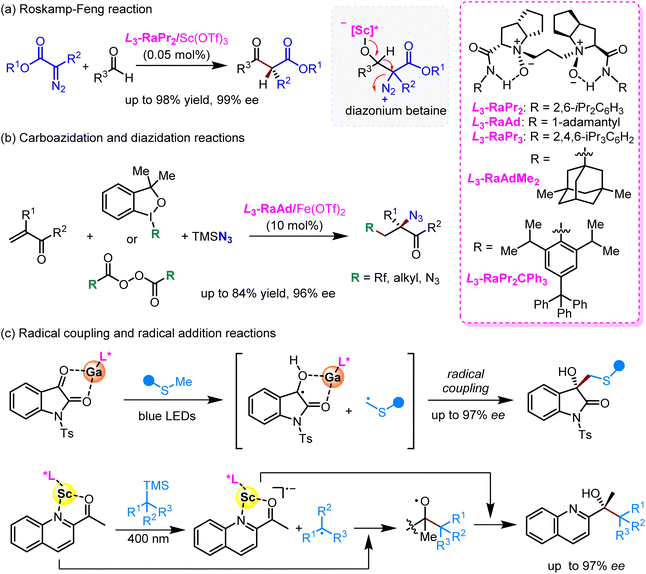
By virtue of the unique flexibility of the Feng ligand design in terms of conformation and coordination chemistry, a variety of Feng ligands have been prepared, characterized, and applied as privileged chiral Lewis acid catalysts in more than 50 asymmetric transformations, including conjugate addition reactions,25 cycloaddition reactions,13 arrangement reactions,26 and so forth (Fig. 2). The success of the Feng ligand design not only represents an invaluable addition to the toolkit of chiral ligand design, but also enhances the stereocontrol of reactions with understood mechanisms and enables unprecedented transformations to occur with high enantioselectivity. Rather than a comprehensive review, only a few examples of prime significance by the Feng group and other groups are illustrated here to highlight the impact of the Feng ligand design.The relatively stable α-diazocarbonyl compound is a useful and versatile building block, and has been used in a broad range of asymmetric transformations that provide direct access to enantioenriched molecules.27,28 Nevertheless, Lewis acid-promoted or catalyzed enantioselective rearrangement reactions still remain challenging. Roskamp and colleagues reported a SnCl2-catalyzed aldol-type reaction of α-diazoesters with aldehydes to give each a diazonium betaine intermediate, which then underwent a concerted an H-shift/N2 extrusion reaction to give a β-keto ester, i.e., the Roskamp reaction.29 Since the asymmetric version of the Roskamp reaction employed α-diazoamides with chiral auxiliaries,30 Feng and coworkers introduced a Feng ligand to Sc(OTf)3 catalysis and achieved the first catalytic enantioselective Roskamp reaction (Scheme 2a).31 This was a landmark reaction in both diazo and malonate chemistry due to (1) side reaction pathways such as epoxidation and aryl group migration being completely shut down using the L3-RaPr2–Sc(OTf)3 complex at a concentration as low as 0.05 mol%, (2) the textbook conclusion about inaccessible α-carbon stereogenic β-keto esters being invalidated,32 (3) this reaction being featured as a “Roskamp–Feng reaction”,33 and (4) a very large number of catalytic enantioselective transformations of α-diazocarbonyls, including homologation with ketones34,35,36–38 and [2,3]-39,40,41 and [3,3]-sigmatropic rearrangements,42 having been developed by virtue of the use of prestigious Feng ligand–metal complexes. More interestingly, a novel metal–carbenoid-combined high-spin Feng ligand–Co(II) catalysis was thereby developed, by which a highly enantioselective vinylogous N–H insertion of secondary aliphatic amines23 and an asymmetric [2 + 1] cycloaddition of thioketones43 were achieved.
The privileged Feng ligand–metal complexes have also been used in elegant radical transformations. In 2021, Feng and coworkers developed an efficient enantioselective radical carboazidation and diazidation reaction of electron-deficient alkenes, in which an L3-RaAd–Fe(OTf)2 complex was used as a redox catalyst for the reductive generation of radical species, as well as azido group transfer (Scheme 2b).44 In direct photochemical reactions, such as [2 + 2] cycloaddition45 and Norrish–Yang type II cyclization,46 use of Feng catalysts yielded high diastereo- and enantioselectivity. More interestingly, Feng and coworkers found that a few Feng ligand–metal-substrate complexes assembled in situ can function as unique photoredox reagents. Upon being excited, these complexes trigger single-electron transfer oxidation of thioethers and silanes, thereby giving nucleophilic radical species for precision radical coupling reactions47 and radical addition to ketones48 in a highly enantioselective manner (Scheme 2c).
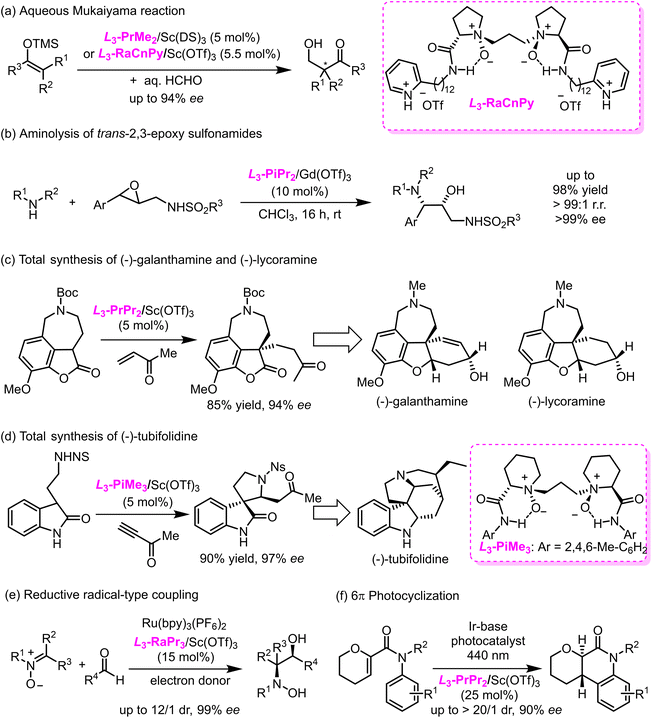
Feng ligands have been commercially available at Sigma-Aldrich and J&K Scientific (Fig. 3) since 2014, which has inspired many other research groups to address issues associated with development of novel reactions and syntheses of molecular complexes. For example, Kobayashi and co-workers leveraged the excellent water tolerance of the L3-PrMe2–Sc(III) complex in aqueous Mukaiyama aldol reactions,49 and they later designed the pH-responsive Feng ligand L3-RaCnPy that functioned as an artificial metalloenzyme (Scheme 3a).50 Yamamoto and colleagues leveraged the L3-PrPr2–Gd(III) and L2-PrPr2–Mg(II) complexes for the first enantioselective aminolysis of aromatic trans-2,3-epoxy sulfonamides (Scheme 3b)51 and amination of β-ketoesters with nitrosocarbonyl compounds generated in situ.52,53 Jia and colleagues demonstrated the superiority of L3-PrPr2–Sc(OTf)3 to “privileged” bifunctional hydrogen-bonding catalysts in the key asymmetric Michael addition reaction of a tricyclic benzofuranone with methyl vinyl ketone towards the total synthesis of (−)-galanthamine and (−)-lycoramine (Scheme 3c).54 Given the unique catalytic activity of L3-PiMe3–Sc(OTf)3, Xie and coworkers developed a large-scale enantioselective tandem Michael addition reaction of oxindoles with alkynones, which afforded the key intermediate for asymmetric total synthesis of monoterpenoidindolealkaloids, including (−)-tubifoline, (−)-tubifolidine, (−)-dehydrotubifoline, and (−)-strychnine (Scheme 3d).55 Moreover, privileged Feng ligand–metal complexes have also provided facile access to a variety of structurally diverse nitrogen-heterocyclic compounds via asymmetric cycloaddition reactions.56,57,58,59 Huang and colleagues60 achieved convergent synthesis of chiral vicinal amino alcohols via an enantioselective reductive radical-type coupling reaction of nitrones with aromatic ketyl radicals generated from photocatalyzed single-electron transfer reduction of carbonyls (Scheme 3e). Paton, Smith, and colleagues61 recently demonstrated that the use of L3-PrPr2–Sc(OTf)3 could lower the triplet energy of acrylanilides by forming complexes with the acrylanilides, to which selective energy transfer from a photosensitizer proceeded, thereby enabling an asymmetric 6π photocyclization reaction (Scheme 3f).
3. Conclusions
In summary, the highly flexible, C2-symmetric chiral N,N′-dioxide amides developed by Feng and coworkers, i.e., Feng ligands, feature tetradentate O-coordination with a broad scope of main-group, transition, and rare-earth metals. The formed chiral metal catalysts have enabled numerous asymmetric transformations that provide invaluable access to many enantio-enriched structurally complex molecules. The Feng ligand has clearly become a highly competent member of the family of privileged ligands. With its ever-increasing popularity and impacts in synthetic organic chemistry, recent examples have opened avenues for controlling the stereoselectivity of radical-related bond-forming processes by virtue of Feng ligand–metal Lewis acid catalysis.44–46,60,61 In the near future, we expect a greatly expanded chemical space of Feng ligands in manipulating such transient intermediates with greener processes, as well as applications in material science.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the National Natural Science Foundation of China (no. 22188101) and the Fundamental Research Funds for the Central Universities (no. WK9990000111).References
-
E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Comprehensive Asymmetric Catalysis, Volumes I to III, Springer, New York, 1999 Search PubMed
.
- M. Reetz, Laboratory Evolution of Stereoselective Enzymes: A Prolific Source of Catalysts for Asymmetric Reactions, Angew. Chem., Int. Ed., 2011, 50, 138–174 CrossRef CAS PubMed
.
- M. Heitbaum, F. Glorius and I. Escher, Asymmetric Heterogeneous Catalysis, Angew. Chem., Int. Ed., 2006, 45, 4732–4762 CrossRef CAS PubMed
.
-
P. I. Dalko, Enantioselective Organocatalysis: Reactions and Experimental Procedures, Wiley-VCH, Weinheim, 2007 Search PubMed
.
-
L. Albrecht, A. Albrecht and L. Dell'Amico, Asymmetric Organocatalysis: New Strategies, Catalysts, and Opportunities, Wiley-VCH, Weinheim, 2023 Search PubMed
.
-
Q.-L. Zhou, Privileged Chiral Ligands and Catalysts, Wiley-VCH, Weinheim, 2011 Search PubMed
.
- F. Fache, E. Schulz, M. L. Tommasino and M. Lemaire, Nitrogen-Containing Ligands for Asymmetric Homogeneous and Heterogeneous Catalysis, Chem. Rev., 2000, 100, 2159–2231 CrossRef CAS PubMed
.
- M. P. Carroll and P. J. Guiry, P, N Ligands in Asymmetric Catalysis, Chem. Soc. Rev., 2014, 43, 819–833 RSC
.
- J.-H. Xie and Q.-L. Zhou, Chiral Diphosphine and Monodentate Phosphorus Ligands on a Spiro Scaffold for Transition-Metal-Catalyzed Asymmetric Reactions, Acc. Chem. Res., 2008, 41, 581–593 CrossRef CAS PubMed
.
- X. H. Liu, S. X. Dong, L. L. Lin and X. M. Feng, Chiral Amino Acids-Derived Catalysts and Ligands, Chin. J. Chem., 2018, 36, 791–797 CrossRef CAS
.
- X. H. Liu, L. L. Lin and X. M. Feng, Chiral N,N′-Dioxide Ligands: Synthesis, Coordination Chemistry and Asymmetric Catalysis, Org. Chem. Front., 2014, 1, 298–302 RSC
.
- X. H. Liu, L. L. Lin and X. M. Feng, Chiral N,N′-Dioxides: New Ligands and Organocatalysts for Catalytic Asymmetric Reactions, Acc. Chem. Res., 2011, 44, 574–587 CrossRef CAS PubMed
.
- X. H. Liu, H. F. Zheng, Y. Xia, L. L. Lin and X. M. Feng, Asymmetric Cycloaddition and Cyclization Reactions Catalyzed by Chiral N,N′-Dioxide−Metal Complexes, Acc. Chem. Res., 2017, 50, 2621–2631 CrossRef CAS PubMed
.
- W. D. Cao, X. H. Liu and X. M. Feng, Chiral N,N′-Dioxide and Their Metal Complexes in Asymmetric Catalysis, Chin. Sci. Bull., 2020, 65, 2941–1951 CrossRef
.
- Z. Wang, X. H. Liu and X. M. Feng, Asymmetric Catalysis Enabled by Chiral N,N’-Dioxide–Nickel(II) Complexes, Aldrichimica Acta, 2020, 53, 3–10 CAS
.
- M.-Y. Wang and W. Li, Feng Ligand: Privileged Chiral Ligand in Asymmetric Catalysis, Chin. J. Chem., 2021, 39, 969–984 CrossRef CAS
.
- J. K. Whitesell, C2 Symmetry and Asymmetric Induction, Chem. Rev., 1989, 89, 1581–1590 CrossRef CAS
.
- G. Helmchen and A. Pfaltz, Phosphinooxazolines–a New Class of Versatile, Modular P,N-Ligands for Asymmetric Catalysis, Acc. Chem. Res., 2000, 33, 336–345 CrossRef CAS PubMed
.
- C. Moberg, The Role of Symmetry in Asymmetric Catalysis, Isr. J. Chem., 2012, 52, 653–662 CrossRef CAS
.
- T. P. Yoon and E. N. Jacobsen, Privileged Chiral Catalysts, Science, 2003, 299, 1691–1693 CrossRef CAS PubMed
.
- Q. H. Li, X. H. Liu, J. Wang, K. Shen and X. M. Feng, Asymmetric Cyanosilylation of Ketones Catalyzed by Novel Chiral N,N’-Dioxide Titanium Complexes, Tetrahedron Lett., 2006, 47, 4011–4014 CrossRef CAS
.
- I. A. O'Neil, N. D. Miller, J. Peake, J. V. Barkley, C. M. R. Low and S. B. Kalindjian, The Novel Use of Proline Derived Amine Oxides in Controlling Amide Conformation, Synlett, 1993, 515–518 CrossRef
.
- R. G. Garvey, J. H. Nelson and R. O. Ragsdale, The Coordination Chemistry of Aromatic Amine N-Oxides, Coord. Chem. Rev., 1968, 3, 375–407 CrossRef CAS
.
- W. Yang, M. P. Pu, X. B. Lin, M. Chen, Y. J. Song, X. H. Liu, Y.-D. Wu and X. M. Feng, Enantioselective Formal Vinylogous N–H Insertion of Secondary Aliphatic Amines Catalyzed by a High-Spin Cobalt(II) Complex, J. Am. Chem. Soc., 2021, 143, 9648–9656 CrossRef CAS PubMed
.
- K. Zheng, X. H. Liu and X. M. Feng, Recent Advances in Metal-Catalyzed Asymmetric 1,4-Conjugate Addition (ACA) of Nonorganometallic Nucleophiles, Chem. Rev., 2018, 118, 7586–7656 CrossRef CAS PubMed
.
- S. X. Dong, X. H. Liu and X. M. Feng, Asymmetric Catalytic Rearrangements with α-Diazocarbonyl Compounds, Acc. Chem. Res., 2022, 55, 415–428 CrossRef CAS PubMed
.
- H. M. L. Davies and R. E. J. Beckwith, Catalytic Enantioselective C−H Activation by Means of Metal−Carbenoid Induced C−H Insertion, Chem. Rev., 2003, 103, 2861–2904 CrossRef CAS PubMed
.
- S.-F. Zhu and Q.-L. Zhou, Transition-Metal-Catalyzed Enantioselective Heteroatom−Hydrogen Bond Insertion Reactions, Acc. Chem. Res., 2012, 45, 1365–1377 CrossRef CAS PubMed
.
- C. R. Holmquist and E. J. Roskamp, A Selective Method for the Direct Conversion of Aldehydes into. beta.-Keto Esters with Ethyl Diazoacetate Catalyzed by Tin(II) Chloride, J. Org. Chem., 1989, 54, 3258–3260 CrossRef CAS
.
- T. Hashimoto, H. Miyamoto, Y. Naganawa and K. Maruoka, Stereoselective Synthesis of α-Alkyl-β-keto Imides via Asymmetric Redox C−C Bond Formation between α-Alkyl-α-diazocarbonyl Compounds and Aldehydes, J. Am. Chem. Soc., 2009, 131, 11280–11281 CrossRef CAS PubMed
.
- W. Li, J. Wang, X. L. Hu, K. Shen, W. T. Wang, Y. Y. Chu, L. L. Lin, X. H. Liu and X. M. Feng, Catalytic Asymmetric Roskamp Reaction of α-Alkyl-α-Diazoesters with Aromatic Aldehydes: Highly Enantioselective Synthesis of α-Alkyl-β-keto Esters, J. Am. Chem. Soc., 2010, 132, 8532–8533 CrossRef CAS PubMed
.
-
J. Clayden, N. Greeves and S. Warren, Organic Chemistry, Oxford University Press, Oxford, 2nd edn, 2012 Search PubMed
.
-
A. Hassner and I. Namboothiri, Organic Syntheses Based on Name Reactions, Elsevier, Oxford, 3rd edn, 2011 Search PubMed
.
- F. Tan, M. P. Pu, J. He, J. Z. Li, J. Yang, S. X. Dong, X. H. Liu, Y.-D. Wu and X. M. Feng, Catalytic Asymmetric Homologation of Ketones with α-Alkyl α-Diazo Esters, J. Am. Chem. Soc., 2021, 143, 2394–2402 CrossRef CAS PubMed
.
- W. Li, X. H. Liu, F. Tan, X. Y. Hao, J. F. Zheng, L. L. Lin and X. M. Feng, Catalytic Asymmetric Homologation of α-Ketoesters with α-Diazoesters: Synthesis of Succinate Derivatives with Chiral Quaternary Centers, Angew. Chem., Int. Ed., 2013, 52, 10883–10886 CrossRef CAS PubMed
.
- W. Li, X. H. Liu, X. Y. Hao, Y. F. Cai, L. L. Lin and X. M. Feng, A Catalytic Asymmetric Ring-Expansion Reaction of Isatins and α-Alkyl-α-Diazoesters: Highly Efficient Synthesis of Functionalized 2- Quinolone Derivatives, Angew. Chem., Int. Ed., 2012, 51, 8644–8647 CrossRef CAS PubMed
.
- W. Li, F. Tan, X. Y. Hao, G. Wang, Y. Tang, X. H. Liu, L. L. Lin and X. M. Feng, Catalytic Asymmetric Intramolecular Homologation of Ketones with α-Diazoesters: Synthesis of Cyclic α-Aryl/Alkyl β-Ketoesters, Angew. Chem., Int. Ed., 2015, 54, 1608–1611 CrossRef CAS PubMed
.
- F. Tan, X. H. Liu, Y. Wang, S. X. Dong, H. Yu and X. M. Feng, Chiral Lewis Acid Catalyzed Reactions of α-Diazoester Derivatives: Construction of Dimeric Polycyclic Compounds, Angew. Chem., Int. Ed., 2018, 57, 16176–16179 CrossRef CAS PubMed
.
- X. B. Lin, Y. Tang, W. Yang, F. Tan, X. H. Liu and X. M. Feng, Chiral Nickel(II) Complex Catalyzed Enantioselective Doyle−Kirmse Reaction of α-Diazo Pyrazoleamides, J. Am. Chem. Soc., 2018, 140, 3299–3305 CrossRef CAS PubMed
.
- X. B. Lin, Z. Tan, W. K. Yang, W. Yang, X. H. Liu and X. M. Feng, Chiral Cobalt(II) Complex Catalyzed Asymmetric [2,3]- Sigmatropic Rearrangement of Allylic Selenides with α-Diazo Pyrazoleamides, CCS Chem., 2021, 3, 1423–1433 CrossRef CAS
.
- X. B. Lin, W. Yang, W. K. Yang, X. H. Liu and X. M. Feng, Asymmetric Catalytic [2,3] Stevens and Sommelet−Hauser rearrangements of α-Diazo Pyrazoleamides with Sulfides, Angew. Chem., Int. Ed., 2019, 58, 13492–13498 CrossRef CAS PubMed
.
- W. Yang, X. B. Lin, Y. Y. Zhang, W. D. Cao, X. H. Liu and X. M. Feng, Nickel(II)-Catalyzed Asymmetric thio-Claisen Rearrangement of α-Diazo Pyrazoleamides with Thioindoles, Chem. Commun., 2020, 56, 10002–10005 RSC
.
- X. B. Lin, M. P. Pu, X. P. Sang, S. Y. Li, X. H. Liu, Y.-D. Wu and X. M. Feng, Asymmetric Catalytic (2 + 1) Cycloaddition of Thioketones to Synthesize Tetrasubstituted Thiiranes, Angew. Chem., Int. Ed., 2022, 61, e202201151 CAS
.
- W. Liu, M. P. Pu, J. He, T. H. Zhang, S. X. Dong, X. H. Liu, Y.-D. Wu and X. M. Feng, Iron-Catalyzed Enantioselective Radical Carboazidation and Diazidation of α,β-Unsaturated Carbonyl Compounds, J. Am. Chem. Soc., 2021, 143, 11856–11863 CrossRef CAS PubMed
.
- H. Yu, S. X. Dong, Q. Yao, L. Chen, D. Zhang, X. H. Liu and X. M. Feng, Enantioselective [2 + 2] Photocycloaddition Reactions of Enones and Olefins with Visible Light Mediated by N,N′-Dioxide-Metal Complexes, Chem. – Eur. J., 2018, 24, 19361–19367 CrossRef CAS PubMed
.
- T. Y. Zhan, L. K. Yang, Q. Y. Chen, R. Weng, X. H. Liu and X. M. Feng, Chiral Lewis Acid-Catalyzed Norrish Type II Cyclization to Synthesize α-Oxazolidinones via Enantioselective Protonation, CCS Chem., 2022 DOI:10.31635/ccschem.022.202202405
, Just Published.
- Z. D. Tan, S. B. Zhu, Y. B. Liu and X. M. Feng, Photoinduced Chemo-, Site- and Stereoselective α-C(sp3) Functionalization of Sulfides, Angew. Chem., Int. Ed., 2022, 61, e202203374 CAS
.
- L. Z. Hou, Y. Q. Zhou, H. Yu, T. Y. Zhan, W. D. Cao and X. M. Feng, Enantioselective Radical Addition to Ketones through Lewis AcidEnabled Photoredox Catalysis, J. Am. Chem. Soc., 2022, 144, 22140–22149 CrossRef CAS PubMed
.
- M. Kokubo, C. Ogawa and S. Kobayashi, Lewis Acid Catalysis in Water with a Hydrophilic Substrate: Scandium-Catalyzed Hydroxymethylation with Aqueous Formaldehyde in Water, Angew. Chem., Int. Ed., 2008, 47, 6909–6911 CrossRef CAS PubMed
.
- T. Kitanosono and S. Kobayashi, Toward Chemistry-Based Design of the Simplest Metalloenzyme-Like Catalyst That Works Efficiently in Water, Chem. – Asian J., 2015, 10, 133–138 CrossRef CAS PubMed
.
- C. Wang and H. Yamamoto, Gadolinium-Catalyzed Regio- and Enantioselective Aminolysis of Aromatic trans-2,3-Epoxy Sulfonamides, Angew. Chem., Int. Ed., 2015, 54, 8760–8763 CrossRef CAS PubMed
.
- B. Maji, M. Baidya and H. Yamamoto, Asymmetric Construction of Quaternary Stereocenters by Magnesium Catalysed Direct Amination of β-Ketoesters using in situ Generated Nitrosocarbonyl Compounds as Nitrogen Sources, Chem. Sci., 2014, 5, 3941–3945 RSC
.
- For another elegant ring-opening reaction of epoxides using Feng ligand, see: L. Yao, Q. Zhu, L. Wei, Z.-F. Wang and C.-J. Wang, Dysprosium(III)-Catalyzed Ring-Opening of meso-Epoxides: Desymmetrization by Remote Stereocontrol in a Thiolysis/Elimination Sequence, Angew. Chem., Int. Ed., 2016, 55, 5829–5833 CrossRef CAS PubMed
.
- L. Li, Q. Yang, Y. Wang and Y. X. Jia, Catalytic Asymmetric Total Synthesis of (−)-Galanthamine and (−)-Lycoramine, Angew. Chem., Int. Ed., 2015, 54, 6255–6259 CrossRef CAS PubMed
.
- W. G. He, J. D. Hu, P. Y. Wang, L. Chen, K. Ji, S. Y. Yang, Y. Li, Z. L. Xie and W. Q. Xie, Highly Enantioselective Tandem Michael Addition of Tryptamine-Derived Oxindoles to Alkynones: Concise Synthesis of Strychnos Alkaloids, Angew. Chem., Int. Ed., 2018, 57, 3806–3809 CrossRef CAS PubMed
.
- M. Boomhoff and C. Schneider, A Novel Three-Component [3 + 2] Cycloannulation Process for the Rapid and Highly Stereoselective Synthesis of Pyrrolobenzoxazoles, Chem. – Eur. J., 2012, 18, 4185–4189 CrossRef CAS PubMed
.
- S. Jayakumar, K. Louven, C. Strohmann and K. Kuma, A Tunable and Enantioselective Hetero-Diels–Alder Reaction Provides Access to Distinct Piperidinoyl Spirooxindoles, Angew. Chem., Int. Ed., 2017, 56, 15945–15949 CrossRef CAS PubMed
.
- Z.-J. Jia, G. Shan, C. G. Daniliuc, A. P. Antonchick and H. Waldmann, Enantioselective Synthesis of the Spirotropanyl Oxindole Scaffold through Bimetallic Relay Catalysis, Angew. Chem., Int. Ed., 2018, 57, 14493–14497 CrossRef CAS PubMed
.
- S. N. Li, H. F. Lu, Z. H. Xu and F. Wei, Ni-Catalyzed Asymmetric Hetero-Diels–Alder Reactions of Conjugated Vinyl Azides: Synthesis of Chiral Azido Polycycles, Org. Chem. Front., 2021, 8, 1770–1774 RSC
.
- C.-X. Ye, Y. Y. Melcamu, H.-H. Li, J.-T. Cheng, T.-T. Zhang, Y.-P. Ruan, X. Zheng, X. Lu and P.-Q. Huang, Dual Catalysis for Enantioselective Convergent Synthesis of Enantiopure Vicinal Amino Alcohols, Nat. Commun., 2018, 9, 410 CrossRef PubMed
.
- B. A. Jones, P. Solon, M. V. Popescu, J.-Y. Du, R. Paton and M. D. Smith, Catalytic Enantioselective 6π Photocyclization of Acrylanilides, J. Am. Chem. Soc., 2023, 145, 171–178 CrossRef CAS PubMed
.
| This journal is © the Partner Organisations 2023 |