Development of an electrochemical sensor based on carbon black for the detection of cannabidiol in vegetable extracts
Marco
Cirrincione
a,
Barbara
Zanfrognini
 b,
Laura
Pigani
b,
Laura
Pigani
 c,
Michele
Protti
c,
Michele
Protti
 a,
Laura
Mercolini
a,
Laura
Mercolini
 a and
Chiara
Zanardi
a and
Chiara
Zanardi
 *bc
*bc
aDepartment of Pharmacy and Biotechnology, Alma Mater Studiorum, Università di Bologna, Via Belmeloro 6, 40126 Bologna, Italy
bInstitute of Organic Synthesis and Photoreactivity (ISOF), National Research Council of Italy (CNR), Via P. Gobetti 101, 40129 Bologna, Italy
cDepartment of Chemical and Geological Sciences, Università di Modena e Reggio Emilia, Via G. Campi 103, 41125 Modena, Italy. E-mail: chiara.zanardi@unimore.it
First published on 23rd October 2020
Abstract
A glassy carbon electrode chemically modified with a carbon black coating is proposed here for the rapid and portable determination of cannabidiol (CBD) in a commercial Cannabis seed oil and in fibre-type Cannabis sativa L. leaves. The mechanism of CBD oxidation was studied in relation to simpler phenyl derivatives bearing the same electroactive group, namely resorcinol and 2-methylresorcinol. These molecules also allowed us to determine the best conditions for the electrochemical detection of CBD, as to the pH value and to the best solvent mixture to use. Carbon black was chosen among nanostructured carbon-based materials owing to its outstanding features as an electrode modifier for analyte detection. The performance of the modified electrode was determined by flow injection analyses of standard solutions of CBD, obtaining a linear correlation between the oxidation current and the analyte concentration; the sensor response is characterised by suitable repeatability and reproducibility. The analysis of commercial products by the standard addition method allowed us to ascertain the accuracy of the sensor for the detection of CBD in real samples.
Introduction
Recreational Cannabis, mainly marijuana, is among the drugs of abuse most frequently used by the young generation.1,2 Its psychotropic action is due the presence of high levels of Δ9-tetrahydrocannabinol (THC), which impairs cognition, psychomotor function, and driving performance. On the other hand, as a consequence of the recognized antioxidant, anti-inflammatory and neuroprotective activities of cannabinoids, many countries have recently legalized the use of fibre-type Cannabis, which is characterized by a low amount of THC (<0.2% w/w) and high levels of the relevant non-psychoactive component of Cannabis, namely cannabidiol (CBD).3 Due to some ascertained beneficial effects on human health, recently shops legally sell oils, edibles and even dried plant parts. Systematic control of all these products now present in the market requires the development of reliable analytical methods to be adapted to a specific frame in which cannabinoid quantification is required.Analytical control of raw materials and commercialized products is mainly performed by instrumental analyses,4 requiring proper laboratory equipment and trained personnel. The low cost, rapidity and simplicity of electrochemical sensors have motivated several groups to develop portable systems for detecting illicit drugs, also including cannabinoids.5–8 However, only very recently the first electrochemical device for the analysis of CBD was proposed,9 although it is not yet applied for the actual quantification of this analyte in commercial products.
Electrochemical detection of cannabinoids is based on the irreversible oxidation of their phenol group.5–8 However, only conventional carbon-based surfaces, namely graphite and glassy carbon, have been used so far for this application. These surfaces generally show rather poor performance, due to the high overvoltages implied in the charge transfer process and to massive electrode fouling. In contrast, it has been recently observed that the peculiar properties of nanosized carbon materials allow the detection of phenol-based species even when present at particularly low concentration values.10–12 Among them, carbon black (CB) has recently emerged as an attractive material for electrochemical sensing since it combines interesting electrocatalytic properties typical of nanosized materials to the cheapness of a carbon material generally used as a filler to improve the mechanical properties of polymer-based composites.13 The interesting electrocatalytic features of this nanomaterial were demonstrated in comparison with different carbon-nanomaterials, namely multiwalled carbon nanotubes14,15 and graphene,15,16 and were ascribed to the high number of defect sites and to the consequent high content of oxidized moieties.17
In this paper, we propose a sensor for the electrochemical detection of CBD in non-psychoactive Cannabis samples and derived products. It consists of a glassy carbon (GC) electrode modified with a CB film. The effectiveness of this electrode coating compared with a bare electrode was ascertained on the basis of preliminary voltammetric measurements recorded on 1,3-dihydroxybenzene (resorcinol, RC) and 2-methylresorcinol (2MRC), i.e. the simplest phenol derivatives possessing the same electroactive group as CBD (Chart 1).
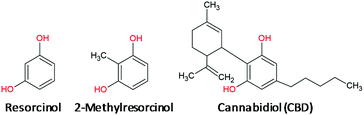 | ||
| Chart 1 Scheme of the chemical structures of CBD and of the relevant simplest phenyl derivatives bearing the same electroactive group. | ||
The reaction mechanism of the different molecules was studied by considering the influence of the solution pH and of the chemical composition of the electrode surface. In particular, two carbon-based electrodes were used, namely GC and polycrystalline graphite (Gr) electrodes, since they are characterized by a higher amount of sp3 and sp2 carbon atoms, respectively. Indeed, the chemical composition of the electrode surface can induce specific interactions with electroactive species in the solution, which are at the basis of the charge transfer process. This may finally affect the oxidation potential and oxidation currents involved in the electrochemical detection. The analytical performance of the sensor for CBD detection in standard solutions as well as in real samples was determined in a flow cell by flow injection analysis (FIA). This approach allows us to propose a sensor for fully automatized analyses of Cannabis sativa samples.
Experimental
Instrumentation
All electrochemical measurements were recorded with an Emstat Multiplexer potentiostat (PalmSens BV), controlled using PSTrace PC software.Voltammetric measurements, namely cyclic voltammetry (CV) and differential pulse voltammetry (DPV), were performed in a conventional three-electrode cell composed of either a GC or a Gr working electrode (Metrohm), a Pt wire as the counter-electrode and a Ag/AgCl/KCl 3 M (Amel) as the reference electrode. Gr and GC working electrodes possess an electroactive area of 4.39 and 3.14 mm2, respectively, as determined on the basis of voltammetric responses collected in a 1 mM [Fe(CN)6]4−, 0.1 M KCl water solution.18
FIAs were performed using the same electrodes in a flow cell (Metrohm) and polarizing the working electrode at a fixed potential. The flow of the electrolytic solution inside the electrochemical cell was achieved with a Miniplus 3 peristaltic pump (Gilson), connected to a Rheodyne valve to introduce a fixed amount of analyte solution with a 100 μL loop. The rotation speed of the pump was fixed at 10 rpm, corresponding to a flow rate of 1.2 mL min−1.
Both GC and Gr electrodes were cleaned by mechanical abrasion with 0.05 μm alumina powder and subsequent immersion in an ultrasonic bath (Bandelin Sonerex) before use. The GC electrode was used as such and after modification with a film of CB-N220 (Cabot Corporation, 19–25 nm diameter, 124 m2 g−1 surface area). The GC/CB modified electrode was obtained by drop-casting, on the GC electrode, a suitable amount of a 1 mg mL−1 suspension of CB obtained in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v DMF (Scharlau, 99.8%) and deionized water solution.17,19
1 v/v DMF (Scharlau, 99.8%) and deionized water solution.17,19
Reagents and solutions
All solutions were prepared starting from a 0.12 M Britton–Robinson buffer (BRB), obtained in deionized water (18 MΩ cm resistivity) by mixing appropriate amounts of H3BO3 (Carlo Erba, 99.8%), CH3COONa (Carlo Erba, 99.5%), and NaH2PO4 (CalbioChem, 98%). The use of the BRB allowed us to perform electrochemical measurements at three different pH values (4.0, 7.0 and 10.0) in solutions containing the same electrolytes. The pH of each solution was adjusted by adding either NaOH (Fisher Chemical, 98.3%) or HCl (Carlo Erba, 37%).RC (≥99%), 2MRC (99%), and CBD (1 mg mL−1 CH3OH solution) were acquired from Sigma-Aldrich. 0.1 mM water solutions of both RC and 2MRC were prepared in BRB at different pH values. Due to the low solubility of cannabinoids in water, solutions of CBD were prepared in a mixed BRB and CH3CN solvent (Zeus, ≥99.5%) in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ratio. For the sake of comparison, analysis of RC was repeated in the same solvent mixture.
2 v/v ratio. For the sake of comparison, analysis of RC was repeated in the same solvent mixture.
Electroanalytical tests
The oxidation mechanism of RC and 2MRC and in particular the reversibility of the electrochemical process were studied in aqueous solutions at different pH values by recording CV responses in the 0.1–0.9 V potential interval, at 0.050 V s−1 scan rate. In order to increase the signal to noise ratio, these analyses were repeated by a pulsed voltammetric technique, namely DPV, by adopting the following parameters: 0.050 V pulse potential, 0.006 V step potential, 0.1 s pulse time, and 0.015 V s−1 potential scan rate. Similar voltammetric experiments were also performed for CBD oxidation in the BRB/CH3CN solvent medium.Electrochemical tests aimed at determining the analytical performance of the CB-based sensor were recorded in FIA. To this aim, a BRB/CH3OH (Sigma Aldrich) mixture was preferred due to rapid ageing of peristaltic pump tubes in CH3CN and to the poorer performance of CB in this organic solvent. The best ratio between water and CH3OH and the best amount of CB for deposition on the GC electrode were chosen on the basis of analysis of a 50 μM RC solution, injected in a BRB (pH = 7.0) flux. The performance of the GC/CB modified electrode in CBD detection was finally determined by depositing 0.08 mg cm−2 of CB on the working electrode surface and by dissolving the analyte in a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v mixture of BRB (pH = 7.0) and CH3OH. The electrode was pre-conditioned in the flux system for 10 min at +0.1 V, in order to eliminate CB particles not perfectly anchored onto the electrode coating. CBD was then analysed in the 0.3–2 mg L−1 concentration interval by polarizing the working electrode at a fixed potential of +0.70 V.
3 v/v mixture of BRB (pH = 7.0) and CH3OH. The electrode was pre-conditioned in the flux system for 10 min at +0.1 V, in order to eliminate CB particles not perfectly anchored onto the electrode coating. CBD was then analysed in the 0.3–2 mg L−1 concentration interval by polarizing the working electrode at a fixed potential of +0.70 V.
Determination of CBD in real samples
The GC/CB electrode was also tested for the quantification of CBD in real samples, consisting of a hemp seed oil enriched with CBD (Easy Oil 0.5, Canapeutical) and of dried leaves taken from a fibre-type Cannabis variant.The analyses were carried out after extraction of the cannabinoids from these samples; for this purpose 1 mL CH3OH was added to 50 mg of sample and mixed in an ultrasonic bath for 5 minutes. The two phases were separated by centrifugation in a Vortex Sprout (Heathrow Scientific). The supernatants obtained from each cycle were reunited and centrifuged with a 6000 Series centrifuge (Centurion) for 10 minutes at 4500 rpm. The extraction procedure was repeated 5 times in order to finally obtain 5 mL of extraction solution in CH3OH. The obtained solution was diluted with BRB solution at pH 7.0 to finally obtain a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v BRB
3 v/v BRB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH mixture. The analysis of CBD in these samples was performed by the standard addition method.
CH3OH mixture. The analysis of CBD in these samples was performed by the standard addition method.
Results and discussion
Electrochemical oxidation of RC and 2MRC in aqueous solution
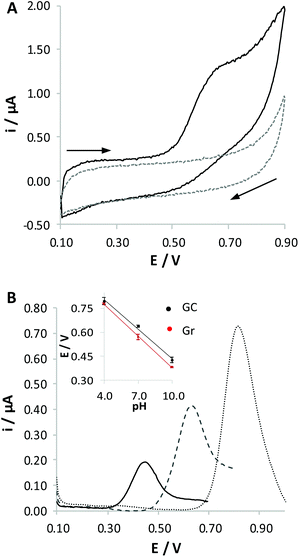
The study of the RC oxidation process was carried out at different pH values (4.0, 7.0 and 10.0) at both Gr and GC electrodes. As expected on the basis of literature reports,20–25 RC is irreversibly oxidized in all the solutions tested (Fig. 1A reports, as an example, the response obtained at pH 7.0). The response is strongly dependent on the pH of the solution (Fig. 1B): the increase of pH induces a shift of the peak potential to lower values, coupled to a strong decrease of the current peak height. Although we cannot find a thermodynamic correlation between the potential shift and the ratio between the numbers of protons and electrons exchanged, Nernst's law being only valid for reversible processes, the fact that the electrochemical process is favoured when increasing the pH of the solution confirms that electrochemical oxidation involves loss of protons, as already reported in the literature;20,25,26 that is, the oxidation process actually involves the deprotonated derivative of phenols. RC oxidation is often described as a two-electron process,24,25,27,28 where both –OH residues of the molecule are oxidized, similarly to what occurs for the relevant structural isomers, i.e. hydroquinone and catechol. This behaviour is in good agreement with the fact that quite similar current peaks are recorded in the electrochemical oxidation of these three species when analysed at the same concentration. However, the exact definition of the nature of the oxidized derivative is, in the case of RC, quite questionable. For this reason, some authors claim that the primary oxidized products undergo a chemical process following the charge transfer, finally leading to a non-conductive polymer chain.25,29,30 Such a process for many aspects recalls what occurs in the case of phenol oxidation.26Voltammetric responses obtained at Gr and at GC electrodes look quite similar. However, the oxidation peaks recorded at the Gr electrode are shifted to lower potential values compared with those measured at the GC electrode (inset in Fig. 1B). This effect can be ascribed to the different chemical nature of these electrode surfaces, since Gr is richer in sp2 hybridized carbon atoms compared with GC. No significant differences in the current peak densities were observed between the two electrode surfaces, but responses recorded at the Gr electrode are more reproducible.
The poor reproducibility obtained for the GC electrode and the higher peak height obtained in the solution at pH 4.0, which is an opposite trend to that expected on the basis of literature articles,21,22,27 suggest the occurrence of massive non-poisoning adsorption phenomena of RC molecules. On the other hand, adsorption of phenol derivatives on carbon-based surfaces is well documented in the literature, so that detection of these species can also be performed by adsorptive stripping voltammetry.31–33 A similar strategy was also applied for the determination of cannabinoids,6,7 to pre-concentrate the analyte on the electrode surface, aiming at increasing sensitivity and reducing the limit of detection.
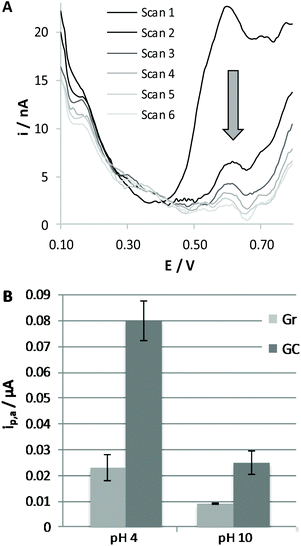
To evaluate the extent of adsorption of RC on varying the nature of the electrode surface and the pH of the solution, we dipped the working electrode, either Gr or GC, in a 0.1 mM RC solution, at a pH of 4.0 or 10.0, for a fixed time of 10 min. The electrode was then carefully washed with water and immersed in BRB solution at pH 7.0 to record the voltammetric response of RC molecules eventually adsorbed on the electrode surface. A typical response obtained at the GC electrode is reported in Fig. 2A. This procedure was repeated three times under the same conditions, in order to verify the significance of the differences observed (see Fig. 2B). As expected, more evident adsorption is obtained in the case of the solution at pH 4.0 and for the GC electrode, confirming that under these conditions adsorption of RC is more significant; we can conclude that adsorption of RC is favoured by the presence of a higher fraction of protonated carboxylic residues and protonated RC molecules. We have to underline that adsorption phenomena generally lead to less reproducible voltammetric responses if the experimental conditions of the electrochemical detection are not well controlled. For this reason, we decided to carry out the subsequent electrochemical tests for 2MRC and CBD oxidation only with the Gr working electrode. Analogously to RC, 2MRC undergoes an irreversible electrooxidation process, shifted to lesser positive potentials the higher the pH of the solution. The height of the anodic voltammetric peak obtained for 2MRC is similar to that recorded for RC, indicating that also this species undergoes a two-electron oxidation process. DPV responses confirm what has already been discussed in the case of RC: the peak intensity increases on lowering the pH of the solution due to the pre-concentration of this species on the electrode surface. This result is expected on the basis of the similar chemical structure of the two molecules. The oxidation potential of 2MRC is, under any conditions, significantly lower than that of RC, due to the presence of the methyl moiety, which induces higher electronic density in the aromatic ring.
Electrochemical oxidation of CBD in an aqueous![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) organic mixture
organic mixture
Due to the limited solubility of cannabinoids in water, the oxidation process of CBD was studied by adding an aliquot of CH3CN to BRB solution. CH3CN was chosen due to its well-known electrochemical inertness, thanks to which it is often preferred among different solvents. CV responses demonstrate that also CBD undergoes an irreversible process, whereas DPV signals recorded at different pH values again indicate that the process is favoured when increasing the pH of the solution (Fig. 3). All these findings lead us to conclude that the electrochemical behaviour of CBD is quite similar to that of RC, involving deprotonation and consecutive oxidation of the phenol residue. Comparison between the electrochemical behaviour of CBD and RC was achieved by recording the voltammetric response in the same BRB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN solution mixture. In this case, only buffer at pH 7.0 was used as the starting aqueous solution since this pH is a compromise between the requirement of recording high current values and that of reducing the adsorption phenomena, which limit the reproducibility of the electrochemical responses. Indeed, experiments carried out under the same conditions of DPV responses in Fig. 2A confirmed that adsorption of RC under these conditions is negligible. Fig. 3 reports the DPV responses obtained for the two molecules in this solvent medium.
CH3CN solution mixture. In this case, only buffer at pH 7.0 was used as the starting aqueous solution since this pH is a compromise between the requirement of recording high current values and that of reducing the adsorption phenomena, which limit the reproducibility of the electrochemical responses. Indeed, experiments carried out under the same conditions of DPV responses in Fig. 2A confirmed that adsorption of RC under these conditions is negligible. Fig. 3 reports the DPV responses obtained for the two molecules in this solvent medium.
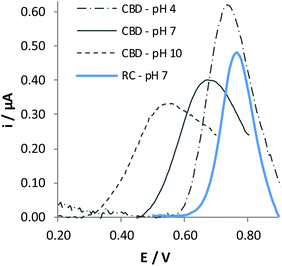
It is evident that the oxidation potential of CBD significantly shifts to less positive potential compared with RC as a consequence of the higher conjugation degree of this molecule. The peak height obtained for CBD oxidation is very close to that obtained for RC oxidation, indicating that both –OH moieties are oxidized at the same potential value.
Unfortunately, the response of CBD oxidation recorded in consecutive potential scans is poorly repeatable, both in the intensity and in the shape of the peak, demonstrating that adsorption phenomena affect the electrochemical process. This drawback forced us to perform the detection of CBD by a different analytical technique. In particular, FIA implies performing analyses by standardizing the contact time of the analyte with the electrode surface, possibly increasing the reproducibility of the responses obtained from different electrodes. In addition, the electrode surface can be somehow cleaned by the flow of the electrolytic solution not containing the analyte for a time long enough to restore the signal to the initial conditions, possibly increasing the repeatability of the electrochemical response. FIA is also suitable for fully automatized analyses, constituting an important added value for the possible application of the sensor in portable devices, easy to be used by any kind of end-users, even those not specifically trained to perform instrumental analysis.
CB modified electrodes for CBD detection
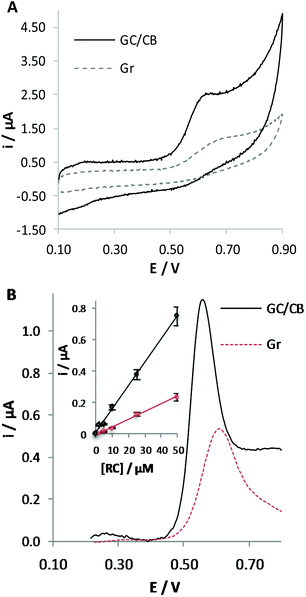
Previous studies led us to conclude that the Gr working electrode favours the oxidation of phenol derivatives since the electrochemical process occurs at lower potential compared with GC, which is richer in sp3-hybridized carbon atoms. Aiming at further improving the performance of the sensor for CBD detection, we decided to modify the surface of the working electrode by a nanosized material possessing a graphitic-like structure. Due to its electrocatalytic properties, already well documented in the literature,11,13,19 our attention was directed to the use of CB. A CB coating was deposited on the surface of GC, since it is one of the most diffused solid carbon electrodes. On the other hand, we could verify that the advantages described hereafter in the use of the CB modified electrodes are also valid when modifying the surface of a commercial Gr electrode.To better demonstrate the advantages in the use of a nanosized material compared with a flat electrode surface possessing similar sp2 carbon atoms, Fig. 4A compares the responses obtained at GC/CB modified and at pristine Gr electrodes in the detection of RC. We could observe that the use of a nanosized material induces a higher current peak and shift of the oxidation process to less positive potential values, indicating the occurrence of electrocatalytic effects. Similar advantages were observed by varying the amount of CB deposited on the electrode surface from 0.032 to 0.128 mg cm−2 (corresponding to a volume of CB suspension ranging from 1 to 4 μl).
The choice of the amount of CB for deposition for the realization of a sensor showing the best performance was made in FIA; this amperometric technique implies the application of a constant potential value while the solution is fluxed in the electrochemical cell. By analysing ten times a 50 μM RC solution at GC/CB electrodes, we could verify that the sensitivity slightly increases up to the deposition of 0.064 mg cm−2 and the best sensor repeatability (RSD = 7.8%) and reproducibility (RSD = 6.0%) were obtained for the deposition of 0.080 mg cm−2. This amount of CB was chosen for the subsequent tests.
Due to the rapid ageing in CH3CN of the peristaltic pump tubes used for FIA configuration and to the poor performance of CB coatings when used in this solvent, we decided to carry out electrochemical analyses in a different organic solvent. Since CH3OH was alternatively used as the organic solvent for liquid chromatographic separations of cannabinoids,34 we decided to use a BRB (pH = 7.0)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH mixture for the subsequent tests. The best amount of CH3OH to be added to the buffer solution was once more chosen on the basis of FIA responses obtained for a 50 μM RC solution. We could observe that both repeatability and reproducibility do not significantly change with the addition of the organic solvent, but the sensitivity decreases when CH3OH exceeds 30% of the total volume of the solution; for this reason, this ratio was chosen for the subsequent analyses. To verify that the advantages in the use of CB modified electrodes are preserved also under these final conditions, a DPV analysis of RC in the BRB (pH 7.0)
CH3OH mixture for the subsequent tests. The best amount of CH3OH to be added to the buffer solution was once more chosen on the basis of FIA responses obtained for a 50 μM RC solution. We could observe that both repeatability and reproducibility do not significantly change with the addition of the organic solvent, but the sensitivity decreases when CH3OH exceeds 30% of the total volume of the solution; for this reason, this ratio was chosen for the subsequent analyses. To verify that the advantages in the use of CB modified electrodes are preserved also under these final conditions, a DPV analysis of RC in the BRB (pH 7.0)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH (30%) at pristine Gr and at GC/CB electrodes was repeated (Fig. 4B). Again, the higher current peak and the shift of the oxidation process to lower potential values testify to the electrocatalytic performance of the material under the adopted conditions.
CH3OH (30%) at pristine Gr and at GC/CB electrodes was repeated (Fig. 4B). Again, the higher current peak and the shift of the oxidation process to lower potential values testify to the electrocatalytic performance of the material under the adopted conditions.
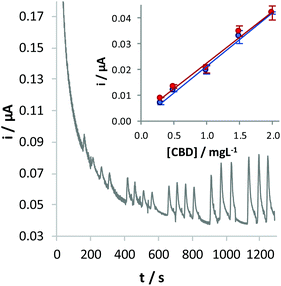
FIA was used to test the performance of the GC/CB electrode for CBD detection (Fig. 5). To this aim, solutions with increasing concentrations of the analyte were injected into the electrochemical cell, obtaining a linear correlation between the oxidation current and the concentration of CBD: i (μA) = 0.206 (±0.013) [CBD] (mg L−1) + 0.007 (±0.015), where within brackets is given the confidence interval (α = 0.05) of the slope and of the intercept, respectively. The limit of detection, calculated as the concentration of CBD corresponding to a signal three times the standard deviation of the blank, is 0.11 mg L−1.
The same measurement was repeated with a different sensor, prepared under similar conditions, by random analysis. Responses obtained by adopting the two conditions led to quite overlapped calibration plots (Fig. 5, inset), as confirmed on the basis of a t-test (p = 0.95) applied on the values of the slope and intercept calculated in the two cases.
Analysis of real samples
A sample of hemp seed oil enriched with CBD, available on the market as a relaxing moisturiser, was tested as the first example of analysis of real samples. The sample was extracted with CH3OH by following an analytical approach already in use for chromatographic analyses.4 The obtained mixture was then diluted 100 times with BRB (pH 7.0) and CH3OH to finally obtain a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v solvent mixture and to fit the concentration of CBD with the linearity range previously obtained (for further details see the Experimental section). The sample was analysed by the standard addition method, obtaining a value of CBD not significantly different from that obtained by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) (see Table 1).
3 v/v solvent mixture and to fit the concentration of CBD with the linearity range previously obtained (for further details see the Experimental section). The sample was analysed by the standard addition method, obtaining a value of CBD not significantly different from that obtained by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) (see Table 1).
| Standard addition | External calibration | LC-MS/MS | |
|---|---|---|---|
| Seed oil | 0.42 ± 0.10% | 0.42 ± 0.11% | 0.480 ± 0.010% |
| Dried leaves | 0.74 ± 0.29% | — | 0.760 ± 0.025% |
During this procedure, we could observe that the slope of the calibration found in the analysis of this hemp oil is not significantly different from that obtained in the analysis of standard CBD solutions, demonstrating a negligible matrix effect. For this reason, the detection of CBD was also repeated by an external calibration, obtaining, also in this case, a value not significantly different from that determined by LC-MS/MS. This method strongly simplifies the analytical procedure, since the quantitation of the analyte can be simply obtained from a single analysis, without any addition of the standard solution.
A fibre-type Cannabis sample of dried leaves was treated by adopting a procedure quite similar to that used for the analysis of hemp oil. Unfortunately, analyses performed with the standard addition method did not lead to accurate evaluation of the amount of CBD contained in this sample. DPV response recorded with this sample (Fig. 6), in fact, evidenced oxidation processes ascribable to different components that may affect the accuracy of the analysis. In particular, vegetable extracts also contain a high amount of cannabidiol acid (CBD-A), which can be easily converted to CBD with a thermic treatment.
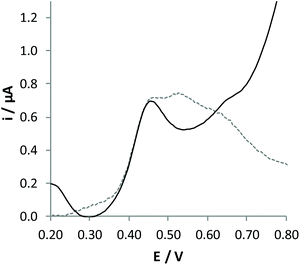 | ||
| Fig. 6 DPV signals recorded at the GC/CB electrode for pristine (dashed line) and pre-treated (solid line) extracts of dried leaves, diluted 21 times with the electrolytic medium. | ||
The pristine Cannabis sativa leaves were heated in an oven first at 110 °C for 15′ and then at 120 °C for 1 hour. In this process, CBD-A is quantitatively transformed into CBD, finally reaching a total concentration of 0.760%, as determined by chromatographic analysis (Table 1). The voltammetric signal obtained from the extract of the decarboxylated sample is reported in Fig. 6 (solid line); it shows a single oxidation response ascribable to CBD oxidation. The quantitative analysis of this extract was performed in FIA by the standard addition method, obtaining a value not significantly different from that determined by conventional chromatographic analysis (Table 1). Indeed, analysis by external calibration is not possible in this case since the internal calibration shows a sensitivity significantly different from that obtained in standard solutions. This demonstrates an evident matrix effect in the analysis of these samples. In addition, the analysis is characterised by lower precision, as evidenced from standard deviation associated with the concentration of CBD found. This demonstrates that some components present in the sample may strongly interact with the surface of the sensor, leading to adsorption phenomena.
Conclusions
We demonstrated that the GC/CB electrode acts as an effective sensor system for CBD quantification in real samples, namely Cannabis seed oils and Cannabis sativa L. leaves. The electrochemical detection of this analyte is based on the oxidation of the di-hydroxyphenyl moiety of CBD molecules, as defined on the basis of electrochemical tests on RC and 2MRC. Electrochemical tests evidenced that the CB coating activates an effective electrocatalytic process, finally shifting the oxidation process to less positive potentials and increasing the sensitivity of the analytical detection.The device was proposed combined with a flow system that on the one hand prevents the poor repeatability and reproducibility of the sensor response and, on the other hand, is suitable for the development of a fully automatable portable sensor system, also exploitable by personnel not specifically trained to perform instrumental analysis. This is perfectly in line with the actual interest in the development of devices for the simple and rapid analysis of drugs of abuse in biofluids for the wide screening of the population.35,36
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the Università di Modena e Reggio Emilia for the financial support in the frame of Interdisciplinary FAR2019: “Novel analytical tools for the determination of cannabinoids in Cannabis sativa L. based products and biological fluids”.Notes and references
- EMCDDA (European Monitoring Centre for Drugs and Drug Addiction), European drug report: trends and developments, Publications office of the European Union, 2019. http://www.emcdda.europa.eu/system/files/publications/11364/20191724_TDAT19001ENN_PDF.pdf, accessed 25 September 2020 Search PubMed.
- UNODC (United Nations Office on Drugs and Crime), World drug report, United Nations publication, 2020. https://wdr.unodc.org/wdr2020/field/WDR20_BOOKLET_1.pdf, accessed 25 September 2020 Search PubMed.
- EMCDDA, Cannabis legislation in Europe: an overview, Lisbon, 2017, http://www.emcdda.europa.eu/publications/adhoc/cannabis-legislation-europe_en, accessed 25 September 2020 Search PubMed.
- M. Protti, V. Brighenti, M. R. Battaglia, L. Anceschi, F. Pellati and L. Mercolini, ACS Med. Chem. Lett., 2019, 10, 539 CrossRef CAS.
- I. Novak, M. Mlakar and Š. Komorsky-Lovrić, Electroanalysis, 2013, 25, 2631 CrossRef CAS.
- M. A. Balbino, M. M. de Menezes, I. C. Eleotério, A. A. Saczk, L. L. Okumura, H. M. Tristão and M. F. de Oliveira, Forensic Sci. Int., 2012, 221, 29 CrossRef CAS.
- R. Nissim and R. G. Compton, Chem. Cent. J., 2015, 9, 41 CrossRef.
- M. Renaud-Young, R. M. Mayall, V. Salehi, M. Goledzinowski, F. J. E. Comeau, J. L. MacCallum and V. I. Birss, Electrochim. Acta, 2019, 307, 351 CrossRef CAS.
- D. López-Iglesias, J. J. García-Guzmán, C. Zanardi, J. M. Palacios-Santander, L. Cubillana-Aguilera and L. Pigani, J. Electroanal. Chem., 2020, 878, 114591 CrossRef.
- K. H. Hui, M. Pumera and A. Bonanni, Chem. – Eur. J., 2015, 21, 11793 CrossRef CAS.
- D. Talarico, F. Arduini, A. Costantino, M. Del Carlo, D. Compagnone, D. Moscone and G. Palleschi, Electrochem. Commun., 2015, 60, 78 CrossRef CAS.
- G. Maccaferri, F. Terzi, Z. Xia, F. Vulcano, A. Liscio, V. Palermo and C. Zanardi, Sens. Actuators, B, 2019, 281, 739 CrossRef CAS.
- F. Arduini, A. Amine, C. Majorani, F. Di Giorgio, D. De Felicis, F. Cataldo, D. Moscone and G. Palleschi, Electrochem. Commun., 2010, 12, 346 CrossRef CAS.
- T. W. B. Lo, L. Aldous and R. G. Compton, Sens. Actuators, B, 2012, 162, 361 CrossRef CAS.
- S. Cinti, F. Arduini, M. Carbone, L. Sansone, I. Cacciotti, D. Moscone and G. Palleschi, Electroanalysis, 2015, 27, 2230 CrossRef CAS.
- C. H. A. Wong, A. Ambrosi and M. Pumera, Nanoscale, 2012, 4, 4972 RSC.
- V. Mazzaracchio, M. R. Tomei, I. Cacciotti, A. Chiodoni, C. Novara, M. Castellino, G. Scordo, A. Amine, D. Moscone and F. Arduini, Electrochim. Acta, 2019, 317, 673 CrossRef CAS.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods, Fundamentals and Applications, John Wiley & Sons Inc, New York, 2nd edn, 2001, p. 813 Search PubMed.
- F. Arduini, C. Zanardi, S. Cinti, F. Terzi, D. Moscone, G. Palleschi and R. Seeber, Sens. Actuators, B, 2015, 212, 536 CrossRef CAS.
- T. A. Enache and A. M. Oliveira-Brett, J. Electroanal. Chem., 2011, 655, 9 CrossRef CAS.
- H. Yin, Q. Zhang, Y. Zhou, Q. Ma, T. Liu, L. Zhu and S. Ai, Electrochim. Acta, 2011, 56, 2748 CrossRef CAS.
- X. Zhou, Z. He, Q. Lian, Z. Li, H. Jiang and X. Lu, Sens. Actuators, B, 2014, 193, 198 CrossRef CAS.
- S. Zhu, W. Gao, L. Zhang, J. Zhao and G. Xu, Sens. Actuators, B, 2014, 198, 388 CrossRef CAS.
- T. S. Sunil Kumar Naik and B. E. Kumara Swamy, J. Electroanal. Chem., 2018, 826, 23 CrossRef CAS.
- A. R. L. da Silva, A. J. dos Santos and C. A. Martínez-Huitle, RSC Adv., 2018, 8, 3483 RSC.
- M. A. Heras, S. Lupu, L. Pigani, C. Pirvu, R. Seeber, F. Terzi and C. Zanardi, Electrochim. Acta, 2005, 50, 1685 CrossRef CAS.
- X. Liu, Y. Li, X. Liu, X. Zeng, B. Kong, S. Luo and W. Wei, J. Solid State Electrochem., 2012, 16, 883 CrossRef CAS.
- M. Khodari, G. A. M. Mersal, E. M. Rabie and H. F. Assaf, Int. J. Electrochem. Sci., 2018, 13, 3460 CrossRef CAS.
- N. Bensalah, A. Gadri, P. Cañizares, C. Sáez, J. Lobato and M. A. Rodrigo, Environ. Sci. Technol., 2005, 39, 7234 CrossRef.
- N. M. M. A. Edris, J. Abdullah, S. Kamaruzaman and Y. Sulaiman, Microchim. Acta, 2019, 186, 261 CrossRef.
- H. Wang, A. Zhang, H. Cui, D. Liu and R. Liu, Microchem. J., 1998, 59, 448 CrossRef CAS.
- L. Pigani, R. Seeber, A. Bedini, E. Dalcanale and M. Suman, Food Anal. Methods, 2014, 7, 754 CrossRef.
- R. Nissim and R. G. Compton, Analyst, 2014, 139, 5911 RSC.
- L. Mercolini, R. Mandrioli, V. Sorella, L. Somaini, D. Giocondi, G. Serpelloni and M. A. Raggi, J. Chromatogr. A, 2013, 1271, 33 CrossRef CAS.
- M. Klimuntowski, M. M. Alam, G. Singh and M. M. R. Howlader, ACS Sens., 2020, 5, 620 CrossRef CAS.
- B. Zanfrognini, L. Pigani and C. Zanardi, J. Solid State Electrochem., 2020, 24, 2603 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |