Catalytic metal-based systems for controlled statistical copolymerisation of lactide with a lactone
E.
Stirling
,
Y.
Champouret
 * and
M.
Visseaux
* and
M.
Visseaux
 *
*
UMR 8181 – UCCS – Unité de Catalyse et de Chimie du Solide, ENSCL, Centrale Lille, Univ. Artois, Univ. Lille, CNRS, F-59000 Lille, France. E-mail: marc.visseaux@ensc-lille.fr; yohan.champouret@univ-lille1.fr
First published on 20th April 2018
Abstract
Polylactide (PLA) is currently considered as a major polymer which could serve as a potential substitute to the widely used petroleum-based plastics. It is typically produced by coordination–insertion polymerisation (Ring-Opening Polymerisation, ROP) of the cyclic ester, lactide (LA), a dimer of lactic acid that is extracted from biomass via biotechnological processing. However, PLA has limitations that hinder its ability to compete with conventional plastics, particularly with regard to its brittle behaviour. Different methodologies have been employed in order to improve the performances of PLA and thereby expand its range of applications. One strategy involves the statistical Ring-Opening coPolymerisation (ROcoP) of LA with another lactone, primarily ε-caprolactone (ε-CL), which enables the inherent properties of each homopolymer to be incorporated within the same polymer chain. Nevertheless, the difference in the reactivity of LA relative to the lactone comonomer and the occurrence of undesired transesterification creates the challenge of producing a strictly random copolymer. Herein, this review aims to present the variety of metal-based catalysts and/or initiators that target the synthesis of statistical copolymers of LA and lactone, under smooth conditions to ensure the best possible controlled polymerisation process.
Introduction
For several decades, plastic materials obtained from non-renewable petroleum and natural gas resources have found remarkable uses in a wide range of applications (e.g. construction, electronics, medicine, transportation, sports equipment, packaging industries and many others), due to their lightness, durability, toughness, ease of processing and resistance to corrosion and chemicals.1 However, the depletion of fossil feedstocks,2 alongside the environmental concerns associated with plastics pollution and waste,3 has driven both the academic and industrial communities to turn to alternatives. Hence, many efforts are currently being made to develop eco-friendly biodegradable materials that can be derived from renewable resources.4 In this respect, synthetic aliphatic polyesters, such as polylactide (PLA), have received a considerable surge in interest for the large-scale replacement of oil-based polymers and plastic products.5 PLA represents one of the most attractive aliphatic polyesters since the lactide (LA) monomer, a cyclic dimer of lactic acid that exists as two isomers L- and D-lactic acid, can be produced abundantly from renewable resources by microbial carbohydrate fermentation6 and its preparation requires less energy than that of oil-based plastics.7 Furthermore, PLA also displays unique properties that make it comparable to commodity polymers like polystyrene (PS) and poly(ethylene terephthalate) (PET).8The most convenient route to prepare high molecular weight PLA, and in a controlled fashion, is through the Ring-Opening Polymerisation (ROP) of LA using metal-based systems, organic catalysts or enzymes. This has led to the development of highly efficient, stereo-selective and living methodologies via ingeniously designed ROP initiators.9 PLA has the advantage of being a biodegradable and biocompatible thermoplastic polymer that can be processed by conventional methods (injection moulding, extrusion, etc. );10 however, its commercialisation has been restricted to food packaging, biomedical and pharmaceutical fields.11 Indeed, PLA suffers from several drawbacks such as (i) brittleness, (ii) poor elasticity, (iii) low thermal stability and (iv) poor gas/water permeability that limit the range of its potential applications.12 To circumvent these problems, modification by plasticisation or blending has been undertaken to improve the permeability and mechanical/thermal properties of PLA.13
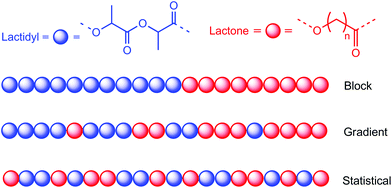
Another strategy, which aims to overcome these drawbacks, involves the Ring-Opening coPolymerisation (ROcoP) of LA with another comonomer. In this case, repeated units of chemically distinct comonomers are covalently linked within the same polymer chain. These copolymers can serve, for example, as compatibilising agents in order to avoid immiscibility, poor interfacial adhesion and chain migration that could occur during blending or plasticisation processes.14 The copolymerisation of LA has been mainly conducted in the presence of lactone, in particular ε-caprolactone (ε-CL), which enables the inherent properties of each homopolymer to be incorporated into the resulting copolymer. The two homopolymers have contrasting physical and thermal properties that make them complementary to each other. For example, polycaprolactone (PCL, Tg(PCL) = −60 °C) exhibits good elasticity and permeability but poor mechanical characteristics (toughness),15 which is the opposite to PLA (Tg(PLA) ≈ 57 °C). Fine-tuning of these properties can a priori be tailored by varying the composition, microstructure (comonomer distribution, stereoregularity), and macromolecular values (molecular weight, dispersity Đ = Mw/Mn). Biodegradable materials with improved properties can thus be produced by copolymerisation of LA with ε-CL, but researchers realised that the difference in reactivity ratios of LA and ε-CL made it difficult to produce a statistical copolymer poly(LA-stat-CL) (Fig. 1). Despite the rate of propagation of ε-CL being typically faster than that of LA in their respective homopolymerisations, the copolymerisation of both monomers often leads to the preferential consumption of LA over ε-CL (i.e. rLA ≫ 1 > rCL). Consequently, the copolymerisation of LA and ε-CL, in most cases, results in the formation of block, poly(LA-block-CL), or gradient (also mentioned as tapered), poly(LA-grad-CL), copolymers (Fig. 1).17 To obtain a random copolymer, the rLA and rCL reactivity ratios must be equal to 1, which will generate average sequence length values of 2 for the caproyl and lactidyl units (LLA = LCL = 2).18 It should be noted that, from the IUPAC recommendations, statistical copolymers refer to copolymers where the sequential distribution of the monomeric unit follows statistical laws (e.g. Markovian statistics) while random copolymers are a special case of statistical copolymers where the sequence distribution obeys Bernoullian statistics (i.e. Markovian statistics of zeroth order).16 Since the 1990s, most LA/ε-CL copolymerisation studies have been carried out at high temperature and/or in bulk and/or at high monomer conversion. This has invariably led to the uncontrolled statistical distribution of both monomers, due to the occurrence of transesterification reactions that reorganise the polymer sequences.19 Nevertheless, despite the uncontrolled nature of these processes, these statistical PLA-based copolymers have shown to display intermediate properties by combining PCL permeability and elasticity in addition to the rather rapid biodegradation of PLA.20 As such, Lu et al. made a comparison of thermomechanical properties of homo-PLA and poly(LA-stat-CL) prepared under similar experimental conditions using stannous octoate at 130 °C in bulk for 48 h with [L-LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Sncat] = 8000
[Sncat] = 8000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and [L-LA]
1 and [L-LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ε-CL]
[ε-CL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Sncat] = 4800
[Sncat] = 4800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3200
3200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. They obtained the following values of Tg = 62 °C, crystallinity = 29.3%, tensile strength = 52.1 MPa, and strain at break = 89.7% for homoPLA, and Tg = 14 °C, amorphous, tensile strength = 20.5 MPa, and strain at break = 541.8% for the copolymer (composition LA/CL = 62/38), emphasising that statistical incorporation of CL in the PLA chain can give materials with varied mechanical properties.20g
1, respectively. They obtained the following values of Tg = 62 °C, crystallinity = 29.3%, tensile strength = 52.1 MPa, and strain at break = 89.7% for homoPLA, and Tg = 14 °C, amorphous, tensile strength = 20.5 MPa, and strain at break = 541.8% for the copolymer (composition LA/CL = 62/38), emphasising that statistical incorporation of CL in the PLA chain can give materials with varied mechanical properties.20g
Herein, this review aims to investigate the range of initiators and/or catalysts by focusing on available metal complexes that target the statistical copolymerisation of lactide with a lactone, primarily ε-CL, under mild conditions to ensure as best as possible a controlled process, with the minimum of undesired transesterification. Other lactones like rac-β-butyrolactone (rac-BL) and δ-valerolactone (VL), which have been scarcely used as comonomers with LA, are also included in this review. Specifically, we will pay attention to the syntheses conducted in solution, with a temperature range of up to 110 °C, which should minimise the occurrence of transesterification. Where possible, the molecular weight, dispersity (Đ = Mw/Mn), reactivity ratios, average sequence lengths of the comonomer units, presence of a Chain-Transfer Agent (CTA) and transition temperature of the resulting copolymer have been displayed.
Poly(lactide-co-glycolide) (PLGA) copolymers and their applications, resulting from LA/glycolide (GA) copolymerisation, have been already subjected to review and will not be discussed here.9a,21 Sequenced copolymerisations will be excluded from the scope of this review to focus on more challenging controlled statistical copolymerisations, which can generate multiple microstructures and thus a large set of properties.
Controlled statistical copolymerisation of lactide with ε-caprolactone
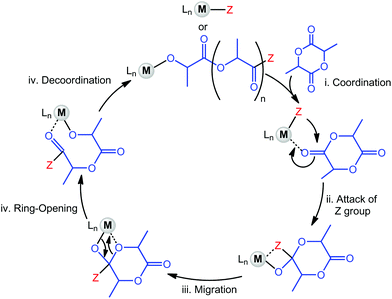
As mentioned previously, the preferred method to prepare polymers of cyclic esters is through the Ring-Opening Polymerisation, typically with the use of a metal complex that may also involve, in some cases, a chain-transfer agent (e.g. alcohol molecule). Currently, the coordination–insertion mechanism is the accepted process in which the reaction occurs, as established through experimental and theoretical evidence (Scheme 1).9The choice of ligands and metal centres can be refined and modified to control the process of statistical copolymerisation of LA and ε-CL. Particular attention has been dedicated to the design of the supporting ligand architecture in order to achieve a truly random copolymer, most notably the sterically hindered salen and its derivatives, phenoxyimine- or other bulky heteroatom-based ligands (vide infra). In addition, important research has been devoted to deciphering the relationship between catalyst structure and its activity, particularly for the use of Al-based complexes. The complications encountered during the attempt to prepare a random copolymer stems from the difficulty of incorporating CL motifs into a PLA growing chain.17 In respect to this, one strategy that has been applied is to alter the structure of the catalyst with the aim to rebalance the reactivity ratios between the two comonomers, either through increasing the activity toward ε-CL or hindering the incorporation of LA units. Mechanistic aspects will be discussed in more details later.
Aluminium catalysts and their ligands
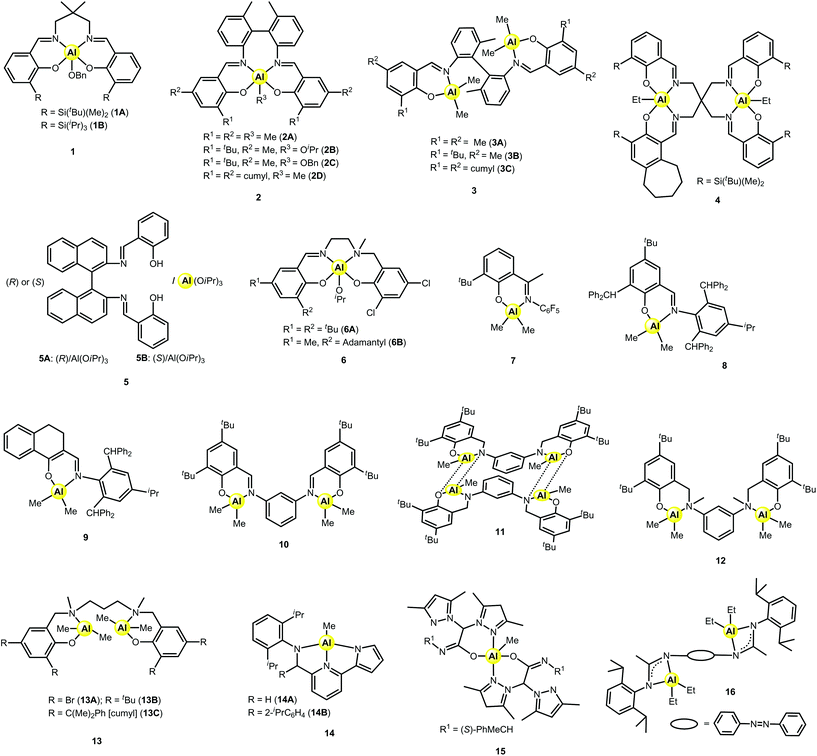
To date, the controlled copolymerisation of LA with ε-CL has been mainly investigated with the help of aluminium-based catalysts. Their efficiency and control can be seen to vary depending on the coordinating ligands. The molecular structures of the aluminium complexes discussed in this section are depicted in Chart 1 and the ROcoP experimental data are gathered in Table 1.| Cat. | [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CL] [CL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Cat] [Cat]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ROH]a [ROH]a |
Solvent (conc.)b | T (°C) | Time (h) | Conv. (%) LA/CL | TOF (h−1) | CL (mol%)c | L LA/LCL | r LA/rCL | Copolymer as claimed by the authors | M n(exp) (g mol−1) | Đ (Mw/Mn) | Transesterification (13C NMR) | T g (°C) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a For the sake of better comparison between all catalysts, only the equivalent monomer feeds are considered, when available. b Total monomer concentration in mol L−1. c Caprolactone units inserted in the copolymer. d As claimed by the authors, 13C NMR not available. e Total polymer yield. f 13C NMR of the copolymer synthesised after 24 h reaction. g DSC of the copolymer synthesised after 48 h reaction. h Total monomer conversion. i In addition of B(C6F5)3. j LA–CL/CL–CL% dyads as measured by 1H NMR. k Copolymerisation with δ-valerolactone, valerolactone units inserted in the copolymer. l Copolymerisation with rac-β-butyrolactone, butyrolactone units inserted in the copolymer. m Hetero-sequences in mol%. n/a = not available. | |||||||||||||||
| 1A | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (rac-LA) 0 (rac-LA) |
Toluene (0.67 M) | 70 | 11 | 95/86 | 5.5 | 47 | 2.0/1.9 | 2.6/1.0 | Gradient | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.10 | n/a | n/a | 22 |
| 1B | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (rac-LA) 0 (rac-LA) |
90 | 10 | 95/97 | 9.6 | 51 | 1.9/1.8 | 0.73/1.0 | Random (alternating tendency) | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.08 | No | −16 | ||
| 2A | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (L-LA) 1 (L-LA) |
Toluene (5 M) | 110 | 4 | 94/87 | 45.2 | 48 | n/a | n/a | Random | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.06 | n/a | n/a | 23 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (rac-LA) 1 (rac-LA) |
5 | 81/74 | 31.0 | 48 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.04 | |||||||||
| 2B | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
Toluene (5 M) | 32 | 86/83 | 5.3 | 49 | 1.91/1.93 | 1.17/0.80 | Purely random | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.04 | No | −9.5 | ||
| 2B | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
Mesitylene (5 M) | 180 | 1.25 | 81/86 | 133.6 | 51 | n/a | n/a | Random | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.09 | n/a | n/a | |
| 2C | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
Toluene (5 M) | 110 | 16 | 85/85 | 10.6 | 50 | n/a | n/a | Random | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.05 | n/a | n/a | |
| 2D | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (L-LA) 1 (L-LA) |
Toluene (5 M) | 110 | 33 | 93/87 | 5.5 | 49 | n/a | n/a | Random | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.05 | n/a | n/a | |
| 3A | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
Toluene (5 M) | 110 | 5 | 82/41 | 24.6 | 33 | 4.51/1.90 | n/a | Gradient | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.14 | No | +12.6 | |
| 3B | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
Toluene (5 M) | 110 | 5 | 69/24 | 18.6 | 26 | n/a | n/a | Gradient | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.22 | n/a | n/a | |
| 3B | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (L-LA) 1 (L-LA) |
Mesitylene (5 M) | 180 | 0.25 | 98/88 | 744 | 47 | n/a | n/a | Gradient | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.64 | n/a | n/a | |
| 3B | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (rac-LA) 0 (rac-LA) |
Toluene (5 M) | 110 | 6.7 | 85/34 | 17.8 | 29 | n/a | n/a | Gradient | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.27 | n/a | n/a | |
| 3C | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (L-LA) 1 (L-LA) |
Toluene (5 M) | 110 | 5 | 74/29 | 20.6 | 28 | n/a | n/a | Gradient | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.22 | n/a | n/a | |
| 4 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (rac-LA) 0 (rac-LA) |
Toluene (0.5 M) | 90 | — | — | — | 51 | 2.2/2.1 | n/a | Gradient | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.66 | No | n/a | 25 |
| 5A | 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
THF (3.75 M) | 80 | 80 | n/a | n/a | n/a | n/a | 4.6/3.1 | Statistical | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.15 | No | n/a | 26 |
| 5B | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
THF (3 M) | 80 | 16 | n/a | n/a | n/a | n/a | 112/7.2 | Gradient/block | 142![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.15 | No | n/a | |
| 6A | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
Toluene (1 M) | 80 | 48 | 81/96 | 1.8 | 57 | 1.9/2.5 | 0.85/2.95 | Random | 8000 | 1.09 | Nod | −17 | 27 |
| 6B | 65/93 | 1.7 | 59 | 1.9/2.2 | n/a | 8400 | 1.09 | −21 | |||||||
| 7 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (rac-LA) 1 (rac-LA) |
Toluene (0.96 M) | 70 | 96 | 52e | 1.0 | 38 | 3.7/1.5 | 5.7–9.8/1.0–1.6 | Random | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.20 | No | −18; 10 | 28 |
96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (L-LA) 1 (L-LA) |
56e | 1.1 | 40 | 1.5/6.5 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.24 | No | +10 | |||||||
| 8 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (rac-LA) 1 (rac-LA) |
Toluene (0.8 M) | 110 | 4 | 95/96 | 47.8 | 50 | 1.9/2.0 | 1.09/1.05 | Random | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.09 | No | −13 | 29 |
| 9 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (rac-LA) 1 (rac-LA) |
2 | 95/97 | 96.0 | 51 | 2.0/2.0 | n/a | Random | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.19 | n/a | n/a | |||
| 10 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (L-LA) 4 (L-LA) |
Toluene (0.5 M) | 70 | 24 | 97/26 | 5.1 | 21 | 9.8/1.2 | n/a | Gradient | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.40 | No | +25.1 | 30 |
| 11 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (L-LA) 4 (L-LA) |
70/24 | 7.8 | 25 | 7.2/1.2 | n/a | Gradient | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.10 | No | +18.2 | ||||
| 12 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (L-LA) 4 (L-LA) |
Toluene (0.5 M) | 91/67 | 6.6 | 42 | 2.6/1.4 | 2.91/0.99 | Tendentiously random | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.21 | No | −3.4 | |||
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 (L-LA) 16 (L-LA) |
Toluene (1 M) | 96/79 | 14.6 | 45 | n/a | n/a | Random | 2900 | 1.25 | n/a | −13.6 | ||||
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 (rac-LA) 16 (rac-LA) |
90/73 | 13.6 | 45 | n/a | n/a | Random | 2700 | 1.19 | n/a | −26.8 | |||||
| 13A | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (L-LA) 4 (L-LA) |
Toluene (2 M) | 70 | 60 | 98/29 | 4.2 | 23 | n/a | n/a | Gradient | 9600 | 1.23 | Medium | −21.8 | 32 |
| 13B | 152 | 94/66 | 2.1 | 41 | Gradient | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.20 | No | −2.4 | ||||||
| 13C | 90 | 95 | 98/53 | 3.2 | 35 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.12 | n/a | n/a | ||||||
| 14A | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (L-LA) 1 (L-LA) |
Toluene (1 M) | 70 | 72 | 87/82 | 4.7 | 55 | 3.2/2.8 | n/a | Random | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.31 | Nod | −5.5 | 33 |
| 14A | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (rac-LA) 1 (rac-LA) |
72 | 83/74 | 4.4 | 53 | 2.5/2.0 | 1.17/1.36 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.13 | No | −3.8 | ||||
| 14B | 120 | 54/43 | 1.6 | 37 | 4.9/1.9 | n/a | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.07 | Nod | −11 | |||||
| 15 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
Toluene (0.9 M) | 66/20 | 3.6 | 13.5 | 9.8/1.1 | 2.8/2.24 | Random | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.68 | No | +36 | 34 | ||
| 16 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
Toluene (0.715 M) | 100 | 24 | 84/19 | 4.3 | 20 | 10.0/1.1 | n/a | Random | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.39 | No | n/a | 35 |
| 17 | 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (L-LA) 2 (L-LA) |
Toluene (33.6 M) | 110 | 2 | 100/99 | 700 | 46 | n/a | n/a | Random | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.06 | Nod | +4.0 | 37 |
| 18A | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
Toluene (0.4 M) | 90 | 144 | 89/87 | 0.6 | 46 | 2.5/2.2 | 1.37/1.15 | Random | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.31 | Nof | +8.0g | 38 |
| 18B | 48 | 18/22 | 0.4 | 50 | 2.3/2.0 | 1.05/0.92 | Random | 3100 | 1.19 | Nod | n/a | ||||
| 19A | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (L-LA) 2 (L-LA) |
Mesitylene (9 M) | 110 | 20 | 96h | 9.6 | 50 | 1.58/1.81 | 0.91/0.93 | Random | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.74 | No | −19.5 | 39 |
| 19B | 21 | 97h | 9.2 | 50 | 1.66/1.75 | 1.07/1.02 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.43 | Little | −30 | |||||
| 19C | 30 | 91h | 6.1 | 50 | 1.63/1.75 | 0.97/0.96 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.44 | −17 | ||||||
| 19D | 30 | 92h | 6.1 | 50 | 1.67/1.73 | 0.95/0.85 | 9900 | 1.29 | −26 | ||||||
| 20 | 173![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 173 173![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (rac-LA) 0 (rac-LA) |
Toluene (0.87 M) | 70 | 24 | 57e | 8.2 | 34 | 3.0/1.8 | n/a | Random blocky | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.50 | Medium | n/a | 40 |
| 21A | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
Toluene (1.04 M) | 100 | 8 | 99/64 | 20.4 | 42 | 2.9/1.7 | n/a | Gradient | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.41 | No | −2 | 41 |
| 21B | 2 | 99/64 | 81.5 | 48 | 2.8/2.2 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.48 | Little | +7 | ||||||
| 21C | 2 | 99/70 | 84.5 | 41 | 3.6/2.2 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.54 | +4 | |||||||
| 22 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
Benzene (3.6 M) | 90 | 96 | 80.4e | 1.7 | 46 | n/a | 14.4/0.36 | Blocky randomised | 5400 | 1.73 | High | n/a | 42 |
| 23 | 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
THF (7.7 M) | 60 | 6 | 51.9e | 25.9 | 7.7 | n/a | n/a | n/a | 8300 | 1.87 | Yesd | n/a | 43 |
| 24A | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
Toluene (2 M) | 70 | 5 | 98/8 | 21.2 | 8 | 14.2/1.2 | n/a | Random | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.26 | No | 52 | 45 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (L-LA) 5 (L-LA) |
24 | 99/47 | 6.1 | 32 | 2.1/1.0 | Random | 5000 | 1.25 | Little | −25; 15 | |||||
| 24B | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
5 | 98/39 | 27.4 | 29 | 3.0/1.2 | Random | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.49 | Little | 58 | ||||
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (L-LA) 5 (L-LA) |
24 | 90/81 | 7.1 | 47 | 1.8/1.6 | Alternating tendency | 6800 | 1.30 | Yes | −20 | |||||
| 24C | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
5 | 99/26 | 25.0 | 20 | 5.9/1.5 | Random | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.60 | Little | 41 | ||||
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (L-LA) 5 (L-LA) |
24 | 89/81 | 7.1 | 48 | 1.4/1.1 | Alternating tendency | 5500 | 1.47 | Yes | n/a | |||||
| 24D | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA) 0 (L-LA) |
5 | 99/28 | 25.4 | 22 | 3.9/1.1 | Random | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.38 | Little | 10 | ||||
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (L-LA) 5 (L-LA) |
24 | 99/69 | 7.0 | 41 | 1.6/1.1 | Alternating tendency | 6700 | 1.29 | Yes | −6 | |||||
| 25 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (rac-LA) 0 (rac-LA) |
Toluene (1.34 M) | 75 | 4 | 39h | 19.5 | 6 | 85/15j | n/a | Random/gradient | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.81 | Little–medium | n/a | 47 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (rac-LA) 1 (rac-LA) |
51h | 25.5 | 13 | 90/10j | 8600 | 1.92 | |||||||||
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (rac-LA) 3 (rac-LA) |
55h | 27.5 | 19 | 91/9j | 5400 | 1.60 | |||||||||
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3i (rac-LA) 3i (rac-LA) |
49h | 24.5 | 5 | 93/7j | 4200 | 1.16 | |||||||||
| 26A | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (rac-LA) 1 (rac-LA) |
Toluene (0.8 M) | 110 | 6 | 92/94 | 31 | 51 | 1.9/2.0 | 2.68/0.29 | Gradient | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.27 | Little | n/a | 29 |
| 26B | 1 | 94/81 | 175 | 46 | 2.4/1.8 | 2.13/0.43 | Gradient | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.06 | No | n/a | ||||
| 26C | 12 | 93/95 | 15.7 | 51 | 2.2/1.8 | 2.29/0.56 | Gradient | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.12 | No | n/a | ||||
| 27 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (L-LA) 3 (L-LA) |
Toluene (1.0 M) | 80 | 2 | 100/100 | 50 | 50 | n/a | n/a | Gradient | 9000 | 1.19 | n/a | n/a | 48 |
| 28 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (rac-LA) 0 (rac-LA) |
Toluene (n/a) | 90 | 22 | 97/19 | 5.3 | n/a | n/a | n/a | Block-type | n/a | n/a | n/a | n/a | 49 |
| 29 | 138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 263 263![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (rac-LA) 0 (rac-LA) |
Toluene (0.8 M) | 70 | 0.5 | 89e | n/a | 15 | n/a | n/a | Gradient | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.52 | Yes | n/a | 51 |
| 3.5 | 82e | 58 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.38 | −3.3 | ||||||||||
| 30 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA)k 0 (L-LA)k |
THF (0.5 M) | 60 | 12 | 61.3e,k | 5.1 | 6.2k | n/a | n/a | n/a | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.3 | n/a | n/a | 53 |
| 31 | 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (rac-LA) 0 (rac-LA) |
CH2Cl2 (0.6 M) | 25 | 1.7 | 100/46 | 129 | n/a | n/a | n/a | Block | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.59 | Yes | n/a | 54 |
150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (rac-LA)l 0 (rac-LA)l |
25 | 1.7 | 100/70l | 150 | n/a | n/a | n/a | Block | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.58 | Yes | n/a | |||
| 32A | 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (rac-LA) 0 (rac-LA) |
Toluene (8.6 M) | 80 | 24 | n/a | n/a | n/a | n/a | n/a | Block | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.61 | n/a | Not observed | 55 |
| 32B | 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (rac-LA)l 0 (rac-LA)l |
80 | 24 | n/a | n/a | n/a | n/a | n/a | Block | n/a | n/a | n/a | |||
| 33 | 111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 108 108![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA)l 0 (L-LA)l |
Toluene (6.6 M) | 50 | 2 | 99/72l | 93.8 | 47l | 45%m | n/a | Gradient | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.27 | Yesd | 24.1 | 56 |
74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (D-LA)l 0 (D-LA)l |
Toluene (4.4 M) | 99/79l | 65.9 | 45l | 40%m | n/a | Gradient | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.24 | Yesd | 22.8 | ||||
88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 88 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (rac-LA)l 0 (rac-LA)l |
Toluene (5.3 M) | 99/66l | 72.6 | 37l | 35%m | n/a | Gradient | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.20 | Yesd | 24.8 | ||||
| 34 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (L-LA)l 0 (L-LA)l |
Toluene (5.0 M) | 70 | 144 | 51/43l | 0.3 | 40l | 56%m | n/a | n/a | 9900 | 1.05 | n/a | n/a | |
| 35 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (L-LA)l 1 (L-LA)l |
Toluene (n/a) | 100 | 24 | 100/100l | 4.2 | 53l | n/a | n/a | Random | n/a | 1.95 | n/a | 31.0 | 57 |
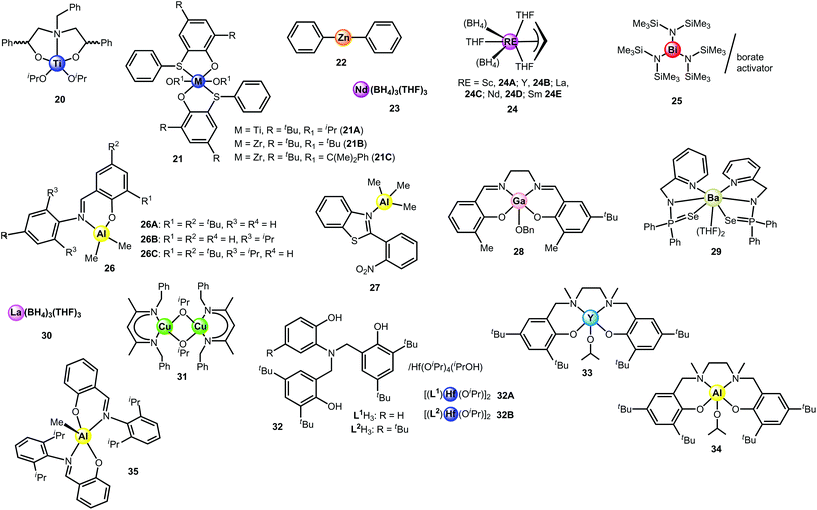
The synthesis of the first controlled random copolymerisation of lactide with ε-caprolactone was reported by Nomura et al. using a salen-type mononuclear aluminium catalyst bearing bulky iPr3Si groups on the ortho position of the phenolate rings, seen in complexes of type 1.22 The two variants of complex 1 successfully copolymerised ε-CL with rac-LA; however, only 1B, bearing the bulkier substituent, was found to produce a copolymer with practically random sequences. The resulting random copolymer had narrow dispersity and the proportion of LA and CL units was nearly equal. The average sequence lengths of the lactidyl and caproyl units were in between 1.7–2.0. Moreover, it was reasoned that by increasing the steric hindrance on the catalyst, the ability of LA to coordinate to the metal centre was reduced, which lowered its reactivity. This enabled the comonomers to be consumed equally with rLA = 0.73 and rCL = 1.09 for complex 1B, thus allowing the synthesis of a random copolymer.
Ma and coworkers achieved practically random copolymers with Bernoullian distributions using salen-type mononuclear aluminium complexes based on a bulky rigid framework comprising a 6,6′-dimethylbiphenyl bridge. This strategy has been employed with the aim to disfavour the LA propagation during the copolymerisation with ε-CL.23 The series of mono- and dinuclear aluminium complexes, 2 (A, B, C, D) and 3 (A, B, C), successfully catalysed the controlled statistical copolymerisation of ε-CL and L- or rac-LA. In the presence of iPrOH, 2A behaved as one of the most active among ROcoP Al-based catalysts (TOF up to 45 h−1), which are generally known to suffer from low activity compared to other metals.24 Complex 2B was less efficient but it reduced the reactivity difference even more between ε-CL and L-LA, resulting in equal consumption of both comonomers throughout the polymerisation. The reactivity ratios, rLA and rCL, were determined to be 1.17 and 0.80, respectively. The propensity of 2B to control the copolymer chain distribution was further established by varying the monomer feed ratio, with the composition of the resulting copolymer mirroring that of the initial ratios. Moreover, the average sequence lengths indicated a random distribution with LLA = 1.91 and LCL = 1.93. Interestingly, the control over the microstructure was maintained at very high temperatures, up to 180 °C, with minor broadening of the dispersity. Catalyst 2C displayed higher activity than 2B at 110 °C, along with a similar control over the microstructure of the copolymer. The presence of a cumyl substituent in 2D was found to be detrimental to the control of the copolymerisation: the incorporation of LA was slightly favoured. The phenoxyimine dinuclear complexes 3A–C turned out to be amongst the most active Al catalysts for L- or rac-LA/ε-CL ROcoP. These metal-based complexes displayed high thermal stability and efficiency even at 180 °C. However, they afforded gradient copolymers since the reduced steric hindrance surrounding the Al centre resulted in higher polymerisation activity toward L-LA.
In a recent study, Pang and coworkers have used bimetallic salen-type complexes of aluminium for the ROP of cyclic esters, and, among them, one complex (4) was subjected to the ROcoP of rac-LA with ε-CL. At 90 °C in toluene, LA/CL content could be adjusted by controlling the feed ratio, since the monomer conversions were complete within the time of the reaction.25 Average sequence lengths of 2.2 (LLA) and 2.1 (LCL) were thus obtained in the case of a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 monomer feed ratio. However, kinetic studies showed that rac-LA was preferentially polymerised during the early stage, and 1H NMR confirmed the formation of a gradient (tapered) copolymer.
50 monomer feed ratio. However, kinetic studies showed that rac-LA was preferentially polymerised during the early stage, and 1H NMR confirmed the formation of a gradient (tapered) copolymer.
The composition of the resulting copolymer can be influenced by the stereochemistry of the coordinating ligands as shown by Duda and coworkers, with salen-type complexes 5.26 The two initiating systems, 5A and 5B (resulting from the in situ reaction of the enantiopure R or S ligand with Al(OiPr)3, respectively), were applied in the copolymerisation of LA and ε-CL. It was determined that by shifting the configuration of the initiator, the reactivity ratios of L-LA and ε-CL could be manipulated. Thus, for 5A, the reactivity ratios were rLA = 4.6 and rCL = 3.1, whereas for 5B, they were rLA = 112 and rCL = 3.1. The microstructures determined for the copolymers were statistical and gradient for R (5A) and S (5B) configurations, respectively.
The aluminium complexes 6 bearing non-chiral salen-type ligands synthesised in the work of Lamberti and coworkers were shown to be active for the statistical copolymerisation of L-LA with ε-CL.27 In the case of 6A, contrary to typical copolymerisation behaviour, the initiator showed a slight preference to the incorporation of the ε-CL monomer rather than the L-LA monomer. For both complexes, when the monomer feed ratio of L-LA and ε-CL was equimolar, the mole ratio of ε-CL was greater than that of the lactide in the copolymer, resulting in the ratio of CL to LA to be around 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40. The average sequence lengths were LLA = 1.9 and LCL = 2.5, and LLA = 1.9 and LCL = 2.2 for 6A and 6B, respectively.
40. The average sequence lengths were LLA = 1.9 and LCL = 2.5, and LLA = 1.9 and LCL = 2.2 for 6A and 6B, respectively.
The phenoxyimine mononuclear aluminium complex 7 was reported by Pappalardo et al. to produce copolymers of ε-CL with LA having a high trend of random character.28 Separate copolymerisations were performed using the lactide monomers L- and rac-LA. The syntheses were conducted in the presence of 1 equiv. methanol, and produced narrow molecular weight distributions of values between 1.06 and 1.24, with low activity (TOF = 1.0–1.1 h−1). The quantity of ε-CL incorporated into the copolymer increased as the feed ratio of ε-CL was increased, with up to 38% (rac-LA) and 40% (L-LA) present in the copolymer chain. Each copolymer contained statistical sequences, with the percentage of hetero-dyads being greater than 50% for all copolymers. Reactivity ratios were estimated by 1H NMR showing that rLA was higher than rCL. Moreover, no transesterification occurred, as observed from most of the 13C NMR spectra, and high molecular weights were reached for all copolymers.
The phenoxyimine Al complex 8 bearing bulky benzyl substituents allowed the controlled random copolymerisation of rac-LA and ε-CL in toluene at 110 °C in a living manner.29 This complex was prepared following the strategy of Nomura to achieve efficient LA/ε-CL copolymerisation catalysts, i.e. by introducing bulky groups on the ortho-position of the phenolate rings. Copolymers produced by 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH were ideal random, due to equal reactivity ratios rLA and rCL (1.09 and 1.05, respectively). Moreover, the absence of transesterification was corroborated with the narrow dispersity observed (Đ = 1.09). In contrast, the phenoxyimine complexes 26 (see further, Chart 3) that bear less bulky ligands than 8 gave gradient copolymers. Preliminary results reported by the same group revealed that the β-ketiminato Al-based complex 9, which also bears bulky substituents, had the same behaviour of simultaneously copolymerising rac-LA and ε-CL at both low and high conversion, thus affording random copolymers.29
BnOH were ideal random, due to equal reactivity ratios rLA and rCL (1.09 and 1.05, respectively). Moreover, the absence of transesterification was corroborated with the narrow dispersity observed (Đ = 1.09). In contrast, the phenoxyimine complexes 26 (see further, Chart 3) that bear less bulky ligands than 8 gave gradient copolymers. Preliminary results reported by the same group revealed that the β-ketiminato Al-based complex 9, which also bears bulky substituents, had the same behaviour of simultaneously copolymerising rac-LA and ε-CL at both low and high conversion, thus affording random copolymers.29
The first immortal copolymerisation of ε-CL and L-LA was conducted by Li, Cui and coworkers through the use of the phenoxyimine dinuclear complex 10.30 For living polymerisation, each molecule of the initiator affords the growth of a single polymer chain, whereas immortal polymerisation involves a metal-based complex in the presence of a large excess of a CTA, which acts as a catalyst and transfer agent, respectively. In this manner, the immortal process allows the growth of several polymer chains per catalyst molecule.31 The multinuclear aluminium complexes 10, 11 and 12 were prepared by reacting AlMe3 with phenoxyimine (10)- and phenoxyamine (11, 12)-type ligands.30 All three complexes successfully initiated the copolymerisation of L-LA with ε-CL in the presence of 4 equiv. iPrOH; however, complexes 11 and 12 failed to incorporate ε-CL in a randomised manner, as indicated by the average lengths of their lactidyl and caproyl units: LLA = 7.2, LCL = 1.2 and LLA = 9.8 and LCL = 1.2, respectively. In contrast, complex 10 was able to statistically copolymerise L-LA and ε-CL (with a feed ratio of [L-LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ε-CL]
[ε-CL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [10]
[10]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [iPrOH] = 100
[iPrOH] = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) with the resulting copolymer containing a quasi-equal composition of both comonomers (CL/LA = 42/58) with average sequence lengths of LLA = 2.6 and LCL = 1.4. Moreover, the immortal capability of the catalytic system was also explored in the presence of iPrOH (from 4 to 16 equiv.) with [L-LA]
4) with the resulting copolymer containing a quasi-equal composition of both comonomers (CL/LA = 42/58) with average sequence lengths of LLA = 2.6 and LCL = 1.4. Moreover, the immortal capability of the catalytic system was also explored in the presence of iPrOH (from 4 to 16 equiv.) with [L-LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ε-CL]
[ε-CL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [10] = 200
[10] = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which saw an improvement from LA/CL = 61/39 (4 equiv. iPrOH) to LA/CL = 55/45. Copolymerisations were also run with 24 and 48 equiv. iPrOH with [L-LA]
1, which saw an improvement from LA/CL = 61/39 (4 equiv. iPrOH) to LA/CL = 55/45. Copolymerisations were also run with 24 and 48 equiv. iPrOH with [L-LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ε-CL]
[ε-CL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [10] = 400
[10] = 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400
400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 800
1 and 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 feed ratios, respectively. The obtained copolymers had narrower molecular weight distributions; however, the level of ε-CL incorporated into the copolymer was slightly reduced compared to that with 16 equiv. iPrOH. Another effect of the immortal polymerisation conditions was the increased rate of the copolymerisation reaction (TOF = 14.6 h−1 in the presence of 16 equiv. iPrOH, and up to 30 h−1 when [L-LA]
1 feed ratios, respectively. The obtained copolymers had narrower molecular weight distributions; however, the level of ε-CL incorporated into the copolymer was slightly reduced compared to that with 16 equiv. iPrOH. Another effect of the immortal polymerisation conditions was the increased rate of the copolymerisation reaction (TOF = 14.6 h−1 in the presence of 16 equiv. iPrOH, and up to 30 h−1 when [L-LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ε-CL]
[ε-CL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [10]
[10]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [iPrOH] is 800
[iPrOH] is 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48). The results were similar when rac-LA was used instead of L-LA.
48). The results were similar when rac-LA was used instead of L-LA.
Dinuclear phenoxyamine Al complexes 13A–C were assessed by Ma for the copolymerisation of L-LA with ε-CL in the presence of 4 equiv. alcohol in a toluene solution.32 A gradient copolymer was obtained with 13A comprising 23% CL inserted. The introduction of bulky groups (tert-butyl, 13B and cumyl, 13C) to the phenyl moieties of the ligand backbone was beneficial to CL incorporation: gradient (tapered) PLA-co-CL copolymers having 41% (13B) and 35% (13C) of incorporated CL could be prepared. Nonetheless, the nature of the alcohol, namely iPrOH, BnOH or tBuOH, had poor influence on the process. Working in melt at elevated temperatures (110 and 140 °C) allowed the formation of copolymers with ca. 50% of CL units. The absence of transesterification was evidenced by 13C NMR for the reactions conducted with 13B in solution as well as in the melt (110 °C). Both the bulkiness of the ligand and the quantity of alcohol were found to be beneficial to CL insertion and to reduce undesired transesterification reactions.
The controlled copolymerisation of L- or rac-LA and ε-CL has also been achieved with non-salen-type aluminium complexes. In the work of Pellecchia and coworkers, the monomethylaluminium complexes bearing a pyrrolylpyridylamido ligand (14A and 14B) were assessed over 3–5 days in the presence of iPrOH with equimolar ratios of the two monomers.33 It was found that between both complexes, only complex 14A promoted the quasi-random copolymerisation of rac-LA and ε-CL, producing copolymers with average sequence lengths equal to LLA = 2.5 and LCL = 2.0. Additionally, the reactivity ratios were calculated, giving rLA = 1.17 and rCL = 1.36, which confirmed the control of the process.
The five-coordinated aluminium complex 15 supported by a chiral acetamidate heteroscorpionate ligand was prepared by Otero, Lara-Sánchez et al.34 The copolymerisation of L-LA with ε-CL was evaluated with the enantiopure complex in toluene at 110 °C and produced copolymers with Mw/Mn values of 1.38, 1.68 and 1.97, depending on the feed ratio. By increasing the [ε-CL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [LA] feed ratio, the percentage of CL in the copolymer rose; however, the overall incorporation of the ε-CL comonomer was low compared to that of the lactide comonomer, with typically 13.5% CL motifs inserted starting from a 100
[LA] feed ratio, the percentage of CL in the copolymer rose; however, the overall incorporation of the ε-CL comonomer was low compared to that of the lactide comonomer, with typically 13.5% CL motifs inserted starting from a 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ratio whilst reaching 19.5% CL in the case of a 200
100 ratio whilst reaching 19.5% CL in the case of a 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 feed ratio. The conversion of LA was substantially higher than the ε-CL conversion, confirmed by the average sequence lengths of monomer units, with LLA values from 18.5 to 6.4 and the values of LCL ranging from 1.0 to 1.9. Evidence for the absence of transesterification was shown through 13C NMR analysis of the copolymers.
100 feed ratio. The conversion of LA was substantially higher than the ε-CL conversion, confirmed by the average sequence lengths of monomer units, with LLA values from 18.5 to 6.4 and the values of LCL ranging from 1.0 to 1.9. Evidence for the absence of transesterification was shown through 13C NMR analysis of the copolymers.
Recently, the amidinate binuclear complex 16 has been successfully assessed for L-LA and ε-CL statistical copolymerisation.35 For equal feed ratios of the comonomers, PLA containing 20% CL was obtained from toluene solution. The average percentage of LA–CL hetero-dyads was found to be higher than 50%, which indicates a statistical repartition of CL into the PLA backbone. This process was exempt from transesterification as confirmed by 13C NMR.
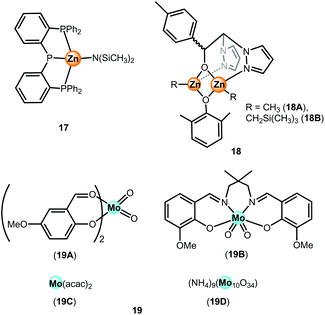
Alternative metal-based catalysts
The development of less toxic, biocompatible metal-based catalysts suitable for the production of polymers with intended applications in biomedical fields would be a desirable improvement from the current ROcoP processes, which primarily involve Sn(Oct)2 and Al initiators.36 These catalysts are displayed in Chart 2 and the ROcoP data are compiled in Table 1.The degradation properties of statistical copolymers with different comonomer ratios prepared with the catalyst (iPr)-PPP-Zn-N(SiHMe2)2 (17, PPP = bis(2-diphenylphosphinophenyl)phosphide, Chart 2) were investigated by Dalmoro et al.37 The pincer-type zinc catalyst effectively performed the statistical copolymerisation between L-LA and ε-CL, with a high percent conversion of both monomers, under experimental conditions close to melt polymerisation ([monomer] = 33.6 M, T = 110 °C). Moreover, the overall compositions of the copolymer chains corresponded to the initial [L-LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ε-CL] feed ratios. However, the synthesis process was not fully controlled, as Mn values were lower than theoretically expected with dispersity values ranging from 1.46–2.06. A full degradation study of these materials concluded that they can be used to design and realise systems with a drug release profile.37b
[ε-CL] feed ratios. However, the synthesis process was not fully controlled, as Mn values were lower than theoretically expected with dispersity values ranging from 1.46–2.06. A full degradation study of these materials concluded that they can be used to design and realise systems with a drug release profile.37b
Chiral dinuclear zinc complexes bearing NNO-scorpionate ligands were synthesised in the work of Honrado et al. and evaluated for the copolymerisation of L-LA with ε-CL.38 It was found that 18A and 18B complexes promoted the synthesis of quasi-random copolymers with reactivity ratios rLA = 1.37, rCL = 1.15, and rLA = 1.05, rCL = 0.92, respectively. For reactions involving equimolar feed ratios, the percentage of ε-CL incorporated into the copolymer was ca. 50%. No transesterification occurred, as shown by the 13C NMR spectrum of the copolymer prepared by complex 18A, which was confirmed by the narrow molecular weight distributions (Đ = 1.23–1.31). However, these initiators displayed very low activity with TOF < 1 h−1.
Dioxomolybdenum complexes 19 were synthesised by Maruta and coworkers for the statistical copolymerisation of L-LA and ε-CL.39 Each molybdenum catalyst performed a copolymerisation with the monomers at an equimolar ratio in the presence of cyclododecanol. Both comonomers, lactide and ε-caprolactone, were equally incorporated into the copolymer chain producing a unit ratio of LA/CL = 50/50. The reactivity ratio were close to that for a true random copolymer (rLA = rCL = 1),18 ranging between rLA = 0.91–1.07 and rCL = 0.85–1.02 for all Mo complexes. Homopolymerisation of L-LA was found to proceed very slowly with complex 19A and faster copolymerisation reaction rates were observed at higher ε-CL amounts in the feed, which may explain the ability of complexes 19 to insert high amounts of ε-CL in the copolymer. On the basis of ε-CL homopolymerisation studies, the 19/cyclododecanol catalytic systems are operating via an activated monomer mechanism, which may likely be also the case in the frame of L-LA and ε-CL copolymerisation.39
Attempted controlled statistical copolymerisation of lactide with ε-caprolactone
In this section, the catalytic systems considered are the ones that, despite targeting a random distribution, achieved copolymers with either a different composition or a statistical character but transesterification reactions are noted. Catalysts based on various electrophilic metals, also including aluminium, are described (Chart 3) and data are displayed in Table 1.The titanium isopropoxide complex 20 supported by a diastereomeric aminodiol ligand was prepared by Peruch and coworkers to catalyse the copolymerisation of rac-LA and ε-CL in diluted toluene at 70 °C.40 The catalyst successfully produced copolymers of statistical distribution; however, transesterification reactions contributed somewhat to the random character. These copolymers can thus be described as statistical copolymers with a block character, due to their long sequence lengths of the LA units. With L-LA instead of rac-LA, the same microstructure was observed, but no transesterification was detected, as this side reaction process is known to be less prominent in this case.40 It is worth noting that the CL % in the copolymer was significantly lower and the transesterification higher when the copolymerisation was carried out in bulk at 130 °C.
Milione and coworkers performed the copolymerisation of L-LA and ε-CL with a series of catalysts comprising group 4 metals (complexes 21A–C).41 For the copolymers synthesised from a molar ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 for L-LA and ε-CL, the titanium and zirconium complexes 21A, 21B and 21C produced copolymers with a LA/CL composition of 58/42, 52/48 and 59/41, respectively, with quite high activity (TOF up to 84.5 h−1) when compared to Al-based co-catalysts (see Table 1). Transesterification side reactions were observed by 13C NMR only for the copolymers of zirconium catalysts 21B and 21C. For the complex 21C, it was thought that the cumyl group may be able to rebalance the reactivity ratios due the increased steric bulk surrounding the metal. Subsequently, the lactide block lengths were slightly shorter than those obtained with 21B. Initial analysis of the 50/50 copolymers achieved by 21A and 21B indicated a statistical distribution within the polymer chain; however, further analysis suggested that the distribution could be gradient.
50 for L-LA and ε-CL, the titanium and zirconium complexes 21A, 21B and 21C produced copolymers with a LA/CL composition of 58/42, 52/48 and 59/41, respectively, with quite high activity (TOF up to 84.5 h−1) when compared to Al-based co-catalysts (see Table 1). Transesterification side reactions were observed by 13C NMR only for the copolymers of zirconium catalysts 21B and 21C. For the complex 21C, it was thought that the cumyl group may be able to rebalance the reactivity ratios due the increased steric bulk surrounding the metal. Subsequently, the lactide block lengths were slightly shorter than those obtained with 21B. Initial analysis of the 50/50 copolymers achieved by 21A and 21B indicated a statistical distribution within the polymer chain; however, further analysis suggested that the distribution could be gradient.
In the work of Contreras, a diphenylzinc initiator 22 was used to perform the copolymerisation of L-LA and ε-CL in the solvent benzene.42 The obtained copolymers were of a block distribution which could become more evenly distributed through transesterification reactions. The zinc initiator was also suggested to catalyse transesterification reactions, as evidenced by the increased randomisation with increased concentration of the initiator. The distribution of the monomers was confirmed by their reactivity ratios where rLA = 14.4, rCL = 0.36, showing a high activity toward L-lactide compared to ε-caprolactone.
The statistical copolymerisation of L-LA and ε-CL was attempted by Nakayama et al. with the non-sterically hindered neodymium trisborohydride 23.43 Despite the catalyst being active toward both ε-CL and L-LA in their homopolymerisation,44 in their copolymerisation the obtained copolymers were primarily composed of lactide. The initial molar feed ratio of both monomers was 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, yet the molar percentage of ε-CL incorporated was only between 2.0 and 7.7%.
50, yet the molar percentage of ε-CL incorporated was only between 2.0 and 7.7%.
A series of mixed allylborohydride rare earth complexes 24 (RE = Sc, Y, La, Nd, Sm) were used as catalysts for the statistical copolymerisation of L-LA with ε-CL in toluene at 70 °C.45 Along the series and starting from an equimolar feed ratio of the two comonomers, the yttrium complex 24B was the most efficient in the absence of a CTA to insert CL in PLA (up to 28.2%). The randomness factor was around 1 (0.77–1.16) and LCL was between 1.1 and 1.5, which indicates homogeneous dispersion of CL motifs into the PLA backbone. When the reactions were carried out in the presence of 5 equiv. BnOH as a CTA, the percentage of CL inserted was largely improved, being over 40% for three complexes: 24B (Y, 47.3%), 24C (La, 47.5%) and 24D (Nd, 40.8%). Dyads of LA–CL type were the major sequences found and the R factor was close to or exceeding the value of 1.5,46 thus establishing a net tendency to an alternating copolymer. However, a little to a non-negligible amount of transesterification was noted in almost all experiments of copolymerisation.
The only study of rac-LA/ε-CL copolymerisation with a bismuth catalyst under mild conditions was realised with Bi[N(SiMe3)2]3 (25).47 Reactions were performed in the presence of the CTA BnOH, the borate co-reagent [HNMe2Ph][B(C6F5)4], or both, to investigate their ability to improve the CL content in the copolymer. This was notable when the quantity of BnOH was increased from 0 to 3 equiv. resulting in an increase of 6% to 19%. Changing the monomers’ ratio ([L-LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ε-CL] = 50
[ε-CL] = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150, 3 equiv. BnOH) increased the ε-CL incorporation significantly with 47% CL, although the number of CL–CL sequences also increased. The addition of [HNMe2Ph][B(C6F5)4] in the polymerisation mixture was not beneficial to increase the CL % but narrower dispersities were noted.
150, 3 equiv. BnOH) increased the ε-CL incorporation significantly with 47% CL, although the number of CL–CL sequences also increased. The addition of [HNMe2Ph][B(C6F5)4] in the polymerisation mixture was not beneficial to increase the CL % but narrower dispersities were noted.
Copolymerisations of rac-LA and ε-CL were carried out with phenoxyimine complexes of aluminium 26A–26C in toluene at 110 °C, bearing less bulky ligands than the parent compound 8 mentioned previously.29 It was found that the rac-LA monomer was more readily converted than ε-CL. The values of the reactivity ratios were calculated accordingly, for 26A–26C, which were found to be in the range of rLA = 2.13–2.68 and rCL = 0.29–0.56. 13C NMR spectra indicated the production of gradient copolymers.
Benzothiazole-supported Al complex 27 was assessed for L-LA and ε-CL copolymerisation by the group of Chen.48 At 80 °C in toluene, gradual consumption of L-LA compared to ε-CL was observed, resulting in the formation of a gradient copolymer. No mention was made of the precise microstructure of the copolymer nor of any occurrence of transesterification.
The catalytic activity of the mononuclear salen-supported gallium complex 28 was screened for the copolymerization of rac-LA and ε-CL ([rac-LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ε-CL]
[ε-CL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [28] = 100
[28] = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 90 °C in toluene by Dagorne, Fliedel and coworkers.49 It was previously shown that the use of gallium species was highly relevant as this metal exhibits better biocompatibility, greater stability in polar/protic medium and, in some cases, higher activity for the ROP of LA compared to its aluminium counterparts.50 In this case, complex 28 showed a greater preference to incorporate LA with respect to CL after 22 h reaction (97% conv. of rac-LA, 19% conv. of ε-CL), producing a block-type PLA-PCL copolymer.
1) at 90 °C in toluene by Dagorne, Fliedel and coworkers.49 It was previously shown that the use of gallium species was highly relevant as this metal exhibits better biocompatibility, greater stability in polar/protic medium and, in some cases, higher activity for the ROP of LA compared to its aluminium counterparts.50 In this case, complex 28 showed a greater preference to incorporate LA with respect to CL after 22 h reaction (97% conv. of rac-LA, 19% conv. of ε-CL), producing a block-type PLA-PCL copolymer.
Starting from a [rac-LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ε-CL] = 34
[ε-CL] = 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 66 mixture, the barium complex 29 supported by a 2-picolylaminodiphenylphosphane chalcogenide [Ph2P(=Se)NHCH2(C5H4N)] ligand51 was found capable of incorporating LA and CL in the ratio 85/15 during the first 30 min, indicating higher reactivity of LA compared to CL, while after a total reaction time of 3 h 30 min, this ratio had advanced to 42/58, accounting for further incorporation of ε-CL monomers. The 13C NMR studies of the copolymer samples indicated that gradient copolymers were formed. Moreover, randomization of microstructures through transesterification was also evidenced.
66 mixture, the barium complex 29 supported by a 2-picolylaminodiphenylphosphane chalcogenide [Ph2P(=Se)NHCH2(C5H4N)] ligand51 was found capable of incorporating LA and CL in the ratio 85/15 during the first 30 min, indicating higher reactivity of LA compared to CL, while after a total reaction time of 3 h 30 min, this ratio had advanced to 42/58, accounting for further incorporation of ε-CL monomers. The 13C NMR studies of the copolymer samples indicated that gradient copolymers were formed. Moreover, randomization of microstructures through transesterification was also evidenced.
Finally, one recent example that also contradicts the generally observed rule that Al-based catalysts are the best for high ε-CL incorporation in lactide–lactone ROcoP is the [8-(2,6-Me-4-H-anilide)-5,6,7-trihydroquinolide]AlMe2 complex. This catalyst was found to be poorly efficient (6.1% ε-CL conversion from a 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 feed) despite being very efficient in both homopolymerisations.52
100 feed) despite being very efficient in both homopolymerisations.52
Attempted statistical copolymerisation of lactide with other lactones
Very few examples have been described regarding the metal-based ROcoP of lactide with lactones other than ε-CL under mild conditions; these complexes are depicted in Chart 3 and the ROcoP experimental data are shown in Table 1.Nakayama et al. used the lanthanum tetrahydroborate complex 30 to perform the copolymerisation of L-lactide with δ-valerolactone (VL),53 since that complex was found to be active for the homopolymerisation of both monomers. However, the unit incorporation of VL into PLA was limited to 6.2% after a 12 hour reaction at 60 °C in THF.
Schaper and Whitehorne observed that under statistical copolymerisation conditions (CH2Cl2 solution, 25 °C) of rac-LA with rac-β-butyrolactone (rac-BL) in the presence of the copper complex 31, complete conversion of rac-LA was achieved before any incorporation of rac-BL. They obtained the same trend with ε-CL and rac-LA copolymerisation.54 Moreover, a lower conversion of lactone (CL or BL) was noticed when compared to lactone homopolymerisations, even after complete conversion of the rac-LA monomer. This was explained, on the basis of NMR (13C and 1H) studies, by the occurrence of transesterification side reactions preferentially with the PLA sequences, which competes with the growing polylactone chain.
The monomers rac-BL and rac-lactide were tentatively copolymerised by the hafnium initiators 32A and 32B in the work of Davidson and coworkers.55 A random copolymer was targeted because their rate of homopolymerisation was similar. However, it was determined from 1H NMR kinetic monitoring that LA was polymerised first, and BL inserted much slower into an M-LA linkage than into an M-BL, hence a block copolymer was produced. In turn, block copolymers could be formed by sequential copolymerisation regardless of the order in which the monomers were added.
Pappalardo and coworkers were interested in modulating the properties of poly(hydroxybutyrate) by copolymerising rac-BL with lactide (L-, D- or rac-LA) by means of salan-based yttrium (33) and aluminium (34) catalysts.56 In toluene at temperatures ranging between 20 and 70 °C, they observed that LA was preferentially incorporated into the copolymer with respect to rac-BL. On the other hand, the yttrium complex gave higher monomer conversions than that of the aluminium one, leading to higher rac-BL incorporation, while both catalysts allowed the preparation of polymers with narrow dispersity. In all cases with both catalysts, gradient copolymers were obtained.
Very recently, diphenoxyimine five-coordinated aluminium complex 35 was briefly evaluated as an initiator for the ROcoP of rac-BL and L-LA in the presence of 1 equiv. BnOH ([rac-LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [rac-BL]
[rac-BL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [35]
[35]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnOH] = 50
[BnOH] = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).571H and 13C NMR analyses revealed the presence of the two monomer units in equal proportion in the resulting copolymer with the characteristic resonances of the LA-BL hetero-dyad. Furthermore, DSC and SEC analyses showed a single glass transition temperature and a monomodal molecular weight distribution, respectively, which confirmed that both monomers are incorporated in the same macromolecular chain.
1).571H and 13C NMR analyses revealed the presence of the two monomer units in equal proportion in the resulting copolymer with the characteristic resonances of the LA-BL hetero-dyad. Furthermore, DSC and SEC analyses showed a single glass transition temperature and a monomodal molecular weight distribution, respectively, which confirmed that both monomers are incorporated in the same macromolecular chain.
Although conducted using a N-heterocyclic carbene and not coordination catalysts, the recent study of Thomas and coworkers into the statistical copolymerisation of rac-BL with LA is worth mentioning herein.58 After 5 h at 60 °C (monomer to catalyst ratio 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the authors obtained a 79% rac-LA/66% rac-BL conversion in toluene, and a 45% L-LA/60% rac-BL conversion in the 1
1), the authors obtained a 79% rac-LA/66% rac-BL conversion in toluene, and a 45% L-LA/60% rac-BL conversion in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 THF/toluene mixture. No details were given regarding the microstructure of the copolymers. Sequential copolymerisation did not succeed in the formation of copolymers.
1 THF/toluene mixture. No details were given regarding the microstructure of the copolymers. Sequential copolymerisation did not succeed in the formation of copolymers.
Mechanistic considerations
As stated previously, the homo-ROP of ε-CL displays a much higher reactivity (in some cases by several orders of magnitude) than that of lactide for most catalytic systems. To account for the reverse higher reactivity of LA vs. ε-CL in statistical ROcoP processes, one may consider the well-known higher coordination ability of the oxophilic metal centre to LA than to ε-CL, due to the two carbonyl groups of the former monomer.22 Moreover, chelation between the last inserted LA monomer unit of the growing PLA chain and the metal catalyst was also originally proposed by the group of Jerome,17c and then evidenced by Lewiński et al.,59 who isolated the first five-membered Al-O-lactate intermediate (chelate species) resulting from the primary insertion of a LA molecule into an aluminium-alkoxide bond, while this chelation could not be identified for CL. This stabilisation effect disfavours the insertion of ε-CL into a metal-PLA growing chain.60 This was often confirmed experimentally, when the propagation of ε-CL was subsequently attempted – with a poor rate of success – to a PLA growing chain on a metal centre.61The first efficient way to thwart the preferential coordination of LA vs. CL was proposed by Nomura, who assumed that the coordination capability of LA could be somewhat reduced by steric encumbrance of the methyl group on LA with bulky substituents on the ligand framework in the coordination sphere of the metal catalyst. This is particularly the case with Al-salen complex 1B.22 In addition to the bulkiness of the salen ligand, the group of Ma used the rigidity of the biphenyl bridge to further reduce the reactivity of L-LA vs. ε-CL, and thus provided extra control toward the ROcoP process.23 This dual strategy of bulkiness/rigidity of the ligands with respect to LA/CL ROcoP was pursued by Shi et al., amongst other authors.29,33 By using the bulky-phenoxyimine and β-ketiminato aluminium complexes 8 and 9, respectively, as catalysts, they were more able to control the random living copolymerisation of rac-LA and ε-CL. Furthermore, modifying the configuration of the active centre was shown by Duda and co-workers to be a way by which the reactivity ratio of ε-CL and enantiopure L-LA could be controlled, thus allowing the formation of a statistical copolymer.26
In a recent and interesting DFT study conducted by Nanok et al. with a series of Al-salen complexes,62 it was computed for homo-ROP that the propagation rate was less favoured for LA than for ε-CL because of the interaction, through several intermolecular bonds, between the incoming monomer LA and the hydrophilic PLA growing chain (van der Waals complex). By contrast, such attractive interactions are not present for the PCL growing chain and the ε-CL monomer (and/or the metal centre) due to the hydrophobic nature of the PCL chain. This in turn reveals that the lower reactivity of the homo-ROP of LA vs. ε-CL is related to the higher stability of the van der Waals complex in the former case. Regarding statistical ROcoP in silico, the authors observed that LA exhibits a higher binding affinity to the propagating species, which severely impedes the access of the ε-CL monomer to the active site (regardless of the previous ring-opened comonomer). In other words, the only chance for an ε-CL monomer to be inserted is clearly connected to the coordination efficiency of LA to the metal active species, hence the bulkiness and the rigidity of ligands. Furthermore, in addition to the metal–monomer and metal–(PLA) growing chain (chelate species) interactions mentioned above, the authors of this theoretical study confirmed by their calculations that the attractive interactions between the growing PLA chain and the incoming monomer also induce the preference of LA to be inserted rather than ε-CL. A way to reduce the reactivity gap between the two monomers, caused by the presence of these two types of interactions with the growing polymer chain – chelate species and van der Waals complex – is then to increase the polymerisation temperature in order to weaken these interactions. Notably, this could be verified experimentally by the work of Cui and coworkers with catalyst 10.30 However, this strategy might not be suitable for all systems, as it may favour detrimental transesterification reaction.
Another way to minimize the gap between the reactivity ratios is to use a catalyst that is highly reactive toward the homo-ROP of ε-CL, with the aim of enabling a more favourable competition vs. LA when ROcoP is considered. For example, the allylbisborohydride complexes of rare-earths 24 have an exceptionally higher activity toward ε-CL (TOF up to 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1) than toward LA (TOF 1300 h−1) in the homo-ROP.45 This is most likely what enabled the achievement of LA/CL copolymers with high CL content and a variety of microstructures, from blocky to statistical and quasi-alternating, despite competition with LA and little steric hindrance in the coordination sphere of the large rare earth metal, which is surrounded by small BH4, allyl and THF ligands.
000 h−1) than toward LA (TOF 1300 h−1) in the homo-ROP.45 This is most likely what enabled the achievement of LA/CL copolymers with high CL content and a variety of microstructures, from blocky to statistical and quasi-alternating, despite competition with LA and little steric hindrance in the coordination sphere of the large rare earth metal, which is surrounded by small BH4, allyl and THF ligands.
The addition of an alcohol as a CTA (i.e. in excess) also appears as a rather efficient way to increase the rate of incorporation of the lower reactive comonomer (in this case ε-CL). Indeed, as already observed for statistical copolymerisation of non-polar monomers under Coordinative Chain-Transfer Polymerisation (CCTP),63 the competition between both monomers and the CTA molecule will contribute to reducing the gap of reactivity between LA vs. ε-CL. This strategy was used with a good degree of success with aluminium,30,32 rare earths45 and bismuth catalysts.47
In terms of catalyst structure–reactivity relationships, Nanok et al. confirmed that electron-capacities and flexibility/rigidity of the ligand backbone in the salen-Al catalysts in their study played a significant role in the rate of the ROP processes involved in homopolymerisations: it is clear that electron-withdrawing groups will assist the incorporation (and hence the polymerisation) of LA and improve the activity of the catalyst toward LA polymerisation, which will in turn be detrimental to the production of truly random ε-CL/LA copolymers in ROcoP.32,62
Conclusions
Although it is still difficult to prepare a truly random poly(lactide-ran-lactone), advances in the controlled statistical Ring-Opening coPolymerisation of lactide with a lactone have been significant in recent years, with many new catalytic systems being synthesised for this purpose. The syntheses of the above complexes were intended to improve the way in which the initiator controls the chain growth and its composition. The aluminium initiator system is one that has been featured heavily within the literature, where it predominantly bears salen ligands and its derivatives. However, there has also been interest toward metals of lower toxicity, such as zinc, where concrete results have been achieved. Moreover, progress is expected to come about with complexes of titanium, rare earths and bismuth, which seem to be on the way toward reaching a controlled ROcoP. It is hoped that these complexes may be the stepping stone toward copolymers that can expand the range of applications of PLA-based copolymers, keeping in mind that the lack of transesterification is an important factor to control the polymerisation, as this enables the production of a copolymer with well-defined structure and properties.In most cases, the reduction of the reactivity gap in ROcoP was realised through reducing the coordination ability of lactide. The influence of the ligand type was then recognised, where, in most cases, it was suggested that a bulkier ligand resulted in rebalancing the reactivity ratios of both comonomers and enabling a better incorporation of the typically less reactive lactone. On the other hand, complexes offering very high reactivity toward ε-CL can be seen as an alternative to attain this goal; however, it is not yet a strategy that has been confirmed.
Undoubtedly, if there is still work to be done in the synthesis of PLA-based copolymers by ROcoP, it is in the search for more efficient catalysts, with development directed toward the use of metals other than aluminium. Another major progression will also be to focus on the generalisation of the ROcoP process in the presence of a CTA in excess, with the aim to attain a fully controlled statistical immortal ROcoP.
Finally, the controlled statistical ROcoP of lactide with lactones other than ε-CL is today very limited. Its extension – following the principles described in the present review with regards to the design of catalysts – to other comonomers such as epoxides or carbonates, those particularly issued from natural resources, is highly desirable for the development of new biobased polymer materials with wide ranges of properties.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The CNRS, the University of Lille and the ERASMUS Exchange Program (grant to E. S.) are greatly acknowledged for financial support.Notes and references
- http://www.plasticseurope.org/use-of-plastics.aspx .
- (a) According to the 2017 BP Statistical Review of World Energy, global total reserve levels, by fossil fuel, are 1139 billion tonnes of coal (extraction would be exhausted in year 2169), 187 trillion cubic meters of natural gas (extraction would be exhausted in year 2068), and 1707 billion barrels of crude oil (extraction would be exhausted in year 2066). These data can be found at https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy.html; (b) W. Keim, CHAPTER 2: Fossil Feedstocks – What Comes After? in Methanol: The Basic Chemical and Energy Feedstock of the Future, ed. M. Bertau, et al., Springer-Verlag, Berlin Heidelberg, 2014 Search PubMed.
- (a) J. Jambeck, R. Geyer, C. Wilcox, T. Siegler, M. Perryman, A. Andrady, R. Narayan and K. Law, Science, 2015, 347, 768 CrossRef CAS PubMed; (b) S. Kubowicz and A. M. Booth, Environ. Sci. Technol., 2017, 51, 12058 CrossRef CAS PubMed.
- (a) M. Vert, I. D. Santos, S. Ponsart, N. Alauzet, J. L. Morgat and J. Coudane, Polym. Int., 2002, 51, 840 CrossRef CAS; (b) R. A. Gross and B. Kalra, Science, 2002, 297, 803 CrossRef CAS PubMed; (c) S. Mecking, Angew. Chem., Int. Ed., 2004, 43, 1078 CrossRef CAS PubMed; (d) L. Nair and C. Laurencin, Prog. Polym. Sci., 2007, 32, 762 CrossRef CAS; (e) V. Siracusa, P. Rocculi, S. Romani and M. Rosa, Trends Food Sci. Technol., 2008, 19, 634 CrossRef CAS; (f) A. Gandini, Macromolecules, 2008, 41, 9491 CrossRef CAS; (g) I. Vroman and L. Tighzert, Materials, 2009, 2, 307 CrossRef CAS; (h) G. E. Luckachan and C. K. S. Pillai, J. Polym. Environ., 2011, 19, 637 CrossRef CAS; (i) N. Berezina and S. M. Martelli, CHAPTER 1: Bio-based Polymers and Materials, in Renewable Resources for Biorefineries, from Book Series, RSC Green Chemistry, 2014, eISBN: 978-1-78262-018-1 Search PubMed; (j) M. A. Hillmyer, Science, 2017, 358, 868 CrossRef CAS PubMed; (k) D. K. Schneiderman and M. A. Hillmyer, Macromolecules, 2017, 50, 3733 CrossRef CAS.
- (a) M. Vert, G. Schwarch and J. Coudane, J. Macromol. Sci., Part A: Pure Appl.Chem., 1995, 32, 787 CrossRef; (b) S. Jacobsen, H. Fritz, P. Degée, P. Dubois and R. Jérôme, Polym. Eng. Sci., 1999, 39, 1311 CrossRef CAS; (c) M. Vert, Biomacromlecules, 2005, 6, 538 CrossRef CAS PubMed; (d) K. Nampoothiri, N. Nair and R. John, Bioresour. Technol., 2010, 101, 8493 CrossRef PubMed; (e) J. Rydz, W. Sikorska, M. Kyulavska and D. Christova, Int. J. Mol. Sci., 2015, 16, 564 CrossRef CAS PubMed; (f) PLA biodegradable polymers, Special Issue on: Polylactide (PLA) Based Biopolymers, in Adv. Drug Delivery Rev, ed. A. J. Domb, R. Langer and A. Basu, 2016, vol. 107, pp. 1–393 Search PubMed.
- (a) Y.-J. Wee, J.-N. Kim and H.-W. Ryu, Food Technol. Biotechnol., 2006, 44(2), 163 CAS; (b) J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539 RSC; (c) C. Gao, C. Ma and P. Xu, Biotechnol. Adv., 2011, 29, 930 CrossRef CAS PubMed; (d) K. Yao and C. Tang, Macromolecules, 2013, 46, 1689 CrossRef CAS; (e) M. Dusselier, P. Van Wouwe, A. Dewaele, E. Makshina and B. F. Sels, Energy Environ. Sci., 2013, 6, 1415 RSC; (f) S. Choi, C. W. Song, J. H. Shin and S. Y. Lee, Metab. Eng., 2015, 28, 223 CrossRef CAS PubMed.
- E. T. H. Vink, K. R. Ràbago, D. A. Glassner and P. R. Gruber, Polym. Degrad. Stab., 2003, 80, 403 CrossRef CAS.
- (a) R. A. Auras, S. P. Singh and J. J. Singh, Packag. Technol. Sci., 2005, 18, 207 CrossRef CAS; (b) A. P. Gupta and V. Kumar, Eur. Polym. J., 2007, 43, 4053 CrossRef CAS; (c) S. Farah, D. G. Anderson and R. Langer, Adv. Drug Delivery Rev., 2016, 107, 367 CrossRef CAS PubMed.
- (a) B. J. O'Keefe, M. A. Hillmyer and W. B. Tolman, J. Chem. Soc., Dalton Trans., 2001, 2215 RSC; (b) A.-C. Albertsson and I. K. Karma, Biomacromolecules, 2003, 4, 1466 CrossRef CAS PubMed; (c) O. Dechy-Cabaret, B. Martin-Vaca and D. Bourissou, Chem. Rev., 2004, 104, 6147 CrossRef CAS PubMed; (d) R. H. Platel, L. M. Hodgson and C. K. Williams, Polym. Rev., 2008, 48(1), 11 CrossRef CAS; (e) C. Jérôme and P. Lecomte, Adv. Drug Delivery Rev., 2008, 60, 1056 CrossRef PubMed; (f) C. A. Wheaton, P. G. Hayes and B. J. Ireland, Dalton Trans., 2009, 4832 RSC; (g) C. M. Thomas, Chem. Soc. Rev., 2010, 39, 165 RSC; (h) M. J. Stanford and A. P. Dove, Chem. Soc. Rev., 2010, 39, 486 RSC; (i) M. H. Chisholm, Pure Appl. Chem., 2010, 82, 1647 CrossRef CAS; (j) S. Slomkowski, S. Penczek and A. Duda, Polym. Adv. Technol., 2014, 25, 436 CrossRef CAS; (k) S. Dagorne, M. Normand, E. Kirillov and J.-F. Carpentier, Coord. Chem. Rev., 2013, 257, 1869 CrossRef CAS; (l) A. Sauer, A. Kapelski, C. Fliedel, S. Dagorne, M. Kol and J. Okuda, Dalton Trans., 2013, 42, 9007 RSC; (m) S. Dagorne and C. Fliedel, Top. Organomet. Chem., 2013, 41, 125 CrossRef CAS; (n) R. Jianming, X. Anguo, W. Hongwei and Y. Hailin, Des. Monomers Polym., 2014, 17, 345 CrossRef.
- E. Castro-Aguirre, F. Iñiguez-Franco, H. Samsudin, X. Fang and R. Auras, Adv. Drug Delivery Rev., 2016, 107, 333 CrossRef CAS PubMed.
- (a) R. E. Drumright, P. R. Gruber and D. E. Henton, Adv. Mater., 2000, 12, 1841 CrossRef CAS; (b) S. Inkinen, M. Hakkarainen, A.-C. Albertsson and A. Södergård, Biomacromolecules, 2011, 12, 523 CrossRef CAS PubMed; (c) L. Xiao, B. Wang, G. Yang and M. Gauthier, Poly(Lactic Acid)-Based Biomaterials: Synthesis, Modification and Applications, Biomedical Science, Engineering and Technology, ed. D. N. Ghista, InTechK, 2012, ISBN: 978-953-307-471-9 Search PubMed; (d) K. Hamad, M. Kaseem, H. W. Yang, F. Deri and Y. G. Ko, eXPRESS Polym. Lett., 2015, 9, 435 CrossRef CAS; (e) B. Tyler, D. Gullotti, A. Mangraviti, T. Utsuki and H. Brem, Adv. Drug Delivery Rev., 2016, 107, 163 CrossRef CAS PubMed; (f) M. Malinconico, E. T. H. Vink and A. Cain, Applications of Poly(lactic Acid) in Commodities and Specialties, in Advances in Polymer Science, Springer, Berlin, Heidelberg, 2017 Search PubMed.
- (a) A. Södergård and M. Stolt, Prog. Polym. Sci., 2002, 27, 1123 CrossRef; (b) R. Auras, B. Harte and S. Selke, Macromol. Biosci., 2004, 4, 835 CrossRef CAS PubMed; (c) M. Källrot, U. Edlund and A.-C. Albertsson, Biomacromolecules, 2007, 8, 2492 CrossRef PubMed; (d) R. Rasal, A. Janorkar and D. Hirt, Prog. Polym. Sci., 2010, 35, 338 CrossRef CAS.
- (a) L. Zhang, C. Xiong and X. Deng, J. Appl. Polym. Sci., 1995, 56, 103 CrossRef CAS; (b) S. Jacobsen and H. Fritz, Polym. Eng. Sci., 1999, 39, 1303 CrossRef CAS; (c) L. Yu, K. Dean and L. Li, Prog. Polym. Sci., 2006, 31, 576 CrossRef CAS; (d) V. Nagarajan, A. Mohanty and M. Misra, ACS Sustainable Chem. Eng., 2016, 4, 2899 CrossRef CAS; (e) R. Muthuraj, M. Misra and A. K. Mohanty, J. Appl. Polym. Sci., 2018, 135, 45726 CrossRef; (f) V. Sangeetha, H. Deka, T. Varghese and S. Nayak, Polym. Compos., 2018, 39, 81 CrossRef CAS.
- (a) C. Koning, M. Van Duin, C. Pagnoulle and R. Jérôme, Prog. Polym. Sci., 1998, 23, 707 CrossRef CAS; (b) H. R. Kricheldorf, Chemosphere, 2001, 43, 49 CrossRef CAS PubMed; (c) L. Calandrelli, A. Calaco, P. Laurienzo, M. Malinconico, O. Petillo and G. Peluso, Biomacromolecules, 2008, 9, 1527 CrossRef CAS PubMed; (d) B. Imre and B. Pukánszky, Eur. Polym. J., 2013, 49, 1215 CrossRef CAS.
- (a) M. A. Woodruff and D. W. Hutmacher, Prog. Polym. Sci., 2010, 35, 1217 CrossRef CAS; (b) A. Arbaoui and C. Redshaw, Polym. Chem., 2010, 1, 801 RSC.
- (a) W. Ring, I. Mita, A. D. Jenkins and N. M. Bikales, Pure Appl. Chem., 1985, 57, 1427 CrossRef CAS; (b) IUPAC Recommendations 2008, in “The Purple Book”, ed. R. G. Jones, E. S. Wilks, W. Val Metanomski, J. Kahovec, M. Hess, R. Stepto and T. Kitayama, RSC Publ., 2nd edn, 2009, ISBN: 978-0-85404-491-7 Search PubMed.
- (a) J. M. Vion, R. Jérôme, P. Teyssié, M. Aubin and R. E. Prudhomme, Macromolecules, 1986, 19, 1828 CrossRef CAS; (b) J. Kasperczyk and M. Bero, Makromol. Chem., 1991, 192, 1777 CrossRef CAS; (c) P. Vanhoorne, P. Dubois, R. Jérôme and P. Teyssié, Macromolecules, 1992, 25, 37 CrossRef CAS; (d) J. Kasperczyk, M. Bero and G. Adamus, Makromol. Chem., 1993, 194, 907 CrossRef; (e) A. Duda, T. Biela, J. Libiszowski, S. Penczek, P. Dubois, D. Mecerreyes and R. Jérôme, Polym. Degrad. Stab., 1998, 59, 215 CrossRef CAS; (f) J. Mosnáček, A. Duda, J. Libiszowski and S. Penczek, Macromolecules, 2005, 38, 2027 CrossRef.
- Y. Gnanou and M. Fontanille, Organic and Physical Chemistry of Polymers, John Wiley & Sons, Hooboken, NJ, 2008, ch. 8.5.11 Search PubMed.
- (a) J. Kasperczyk and M. Bero, Makromol. Chem., 1993, 194, 913 CrossRef CAS; (b) M. Bero and J. Kasperczyk, Macromol. Chem. Phys., 1996, 196, 3251 CrossRef; (c) I. Kreiser-Saunders and H. R. Kricheldorf, Macromol. Chem. Phys., 1998, 199, 1081 CAS; (d) H. R. Kricheldorf, K. Bornhorst and H. Hachmann-Thiessen, Macromolecules, 2005, 38, 5017 CrossRef CAS.
- (a) R. Wada, S.-H. Hyon, T. Nakamura and Y. Ikada, Pharm. Res., 1991, 8, 1292 CrossRef CAS PubMed; (b) M. P. Hiljanen-Vainio, P. A. Orava and J. V. Seppälä, J. Biomed. Mater. Res., 1997, 34, 39 CrossRef CAS PubMed; (c) G. Kister, G. Cassanas, M. Bergounhon, D. Hoarau and M. Vert, Polymer, 2000, 41, 925 CrossRef CAS; (d) M. F. Meek, K. Jansen, R. Streendam, W. van Oeveren, P. B. van Wachem and M. J. A. van Luyn, J. Biomed. Mater. Res., Part A, 2004, 68, 43 CrossRef PubMed; (e) S. I. Jeong, B.-S. Kim, Y. M. Lee, K. J. Ihn, S. H. Kim and Y. H. Kim, Biomacromolecules, 2004, 5, 1303 CrossRef CAS PubMed; (f) F. Faÿ, E. Renard, V. Langlois, I. Linossier and K. Vallée-Rechel, Eur. Polym. J., 2007, 43, 4800 CrossRef; (g) X. Lu, Z. Sun and W. Cai, Phys. Scr., 2007, T129, 231 CrossRef CAS; (h) J. Fernández, A. Etxeberria, J. M. Ugartemendia, S. Petisco and J.-R. Sarasua, J. Mech. Behav. Biomed. Mater., 2012, 12, 29 CrossRef PubMed; (i) J. Fernández, A. Etxeberria and J. R. Sarasua, J. Mech. Behav. Biomed. Mater., 2012, 9, 100 CrossRef PubMed; (j) J. Fernández, E. Meaurio, A. Chaos, A. Etxeberria, A. Alonso-Varona and J. R. Sarasua, Polymer, 2013, 54, 2621 CrossRef; (k) J. Fernández, A. Larrañaga, A. Etxeberria, W. Wang and J. R. Sarasua, J. Biomed. Mater. Res., Part A, 2014, 102, 3573 CrossRef PubMed.
- (a) X. S. Wu, Synthesis and properties of biodegradable lactic/glycolic acid polymers, in Encyclopedic Handbook of Biomaterials and Bioengineering, ed. D. L. Wise, et al., Marcel Dekker, New York, 1995, p. 1015 Search PubMed; (b) H. K. Makadia and S. J. Siegel, Polymers, 2011, 3, 1377 CrossRef CAS PubMed; (c) P. Gentile, V. Chiono, I. Carmagnola and P. V. Hantton, Int. J. Mol. Sci., 2014, 15, 3640 CrossRef CAS PubMed; B. Azimi, P. Nourpanah, M. Rabiee and S. Arbab, J. Eng. Fibers Fabr., 2014, 9, 47 Search PubMed; (d) L. Naves, C. Dhand, L. Amlmeida, L. Rajamani, S. Ramakrishna and G. Soares, Prog. Biomater., 2017, 6, 1 CrossRef PubMed.
- N. Nomura, A. Akita, R. Ishii and M. Mizuno, J. Am. Chem. Soc., 2010, 132, 1750 CrossRef CAS PubMed.
- C. Kan and H. Ma, RSC Adv., 2016, 6, 47402 RSC.
- N. Maudoux, T. Roisnel, V. Dorcet, J.-F. Carpentier and Y. Sarazin, Chem. – Eur. J., 2014, 20, 6131 CrossRef CAS PubMed.
- Z. Sun, R. Duan, J. Yang, H. Zhang, S. Li, X. Pang, W. Chen and X. Chen, RSC Adv., 2016, 6, 17531 RSC.
- M. Florczak and A. Duda, Angew. Chem., Int. Ed., 2008, 47, 9088 CrossRef CAS PubMed.
- A. Pilone, N. De Maio, K. Press, V. Venditto, D. Pappalardo, M. Mazzeo, C. Pellecchia, M. Kol and M. Lamberti, Dalton Trans., 2015, 44, 2157 RSC.
- D. Pappalardo, L. Annunziata and C. Pellecchia, Macromolecules, 2009, 42, 6056 CrossRef CAS.
- T. Shi, W. Luo, S. Liu and Z. Li, J. Polym. Sci., Part A: Polym. Chem., 2018, 56, 611 CrossRef CAS.
- L. Li, B. Liu, D. Liu, C. Wu, S. Li, B. Liu and D. Cui, Organometallics, 2014, 33, 6474 CrossRef CAS.
- (a) S. Inoue, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 2861 CrossRef CAS; (b) N. Ajellal, J.-F. Carpentier, C. Guillaume, S. Guillaume, M. Helou, V. Poirier, Y. Sarazin and A. Trifonov, Dalton Trans., 2010, 39, 8363 RSC.
- Y. Wang and H. Ma, Chem. Commun., 2012, 48, 6729 RSC.
- G. Li, M. Lamberti, D. Pappalardo and C. Pellecchia, Macromolecules, 2012, 45, 8614 CrossRef CAS.
- J. A. Castro-Osma, C. Alonso-Moreno, I. Márquez-Segovia, A. Otero, A. Lara-Sánchez, J. Fernández-Baeza, A. M. Rodríguez, L. F. Sánchez-Barba and J. C. García-Martínez, Dalton Trans., 2013, 42, 9325 RSC.
- D. Osorio Melendez, J. A. Castro-Osma, A. Lara-Sanchez, R. S. Rojas and A. Otero, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 2397 CrossRef CAS.
- See for example: (a) R. Mazarro, A. de Lucas, I. Gracia and J. F. Rodríguez, J. Biomed. Mater. Res., Part B, 2008, 85, 196 CrossRef PubMed; (b) D. J. Darensbourg and O. Karroonnirum, Macromolecules, 2010, 43, 8880 CrossRef CAS; (c) V. Balasanthiran, T. L. Beilke and M. H. Chisholm, Dalton Trans., 2013, 42, 9274 RSC.
- (a) I. D'Auria, M. Lamberti, M. Mazzeo, S. Milione, G. Roviello and C. Pellecchia, Chem. – Eur. J., 2012, 18, 2349 CrossRef PubMed; (b) A. Dalmoro, A. A. Barba, M. Lamberti, M. Mazzeo, V. Venditto and G. Lamberti, J. Mater. Sci., 2014, 49, 5986 CrossRef CAS.
- M. Honrado, A. Otero, J. Fernández-Baeza, L. F. Sánchez-Barba, A. Garcés, A. Lara-Sánchez and A. M. Rodríguez, Organometallics, 2016, 35, 189 CrossRef CAS.
- Y. Maruta and A. Abiko, Polym. Bull., 2014, 71, 989 CrossRef CAS.
- D. Dakshinamoorthy and F. Peruch, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 2161 CrossRef CAS.
- (a) F. D. Monica, E. Luciano, A. Buonerba, A. Grassi, S. Milione and C. Capacchione, RSC Adv., 2014, 4, 51262 RSC; (b) F. D. Monica, E. Luciano, G. Roviello, A. Grassi, S. Milione and C. Capacchione, Macromolecules, 2014, 47, 2830 CrossRef.
- J. Contreras and D. Dávila, Polym. Int., 2006, 55, 1049 CrossRef CAS.
- Y. Nakayama, S. Okuda, H. Yasuda and T. Shiono, React. Funct. Polym., 2007, 67, 798 CrossRef CAS.
- S. M. Guillaume, M. Schappacher and A. Soum, Macromolecules, 2003, 36, 54 CrossRef CAS.
- S. Fadlallah, J. Jothieswaran, F. Capet, F. Bonnet and M. Visseaux, Chem. – Eur. J., 2017, 23, 15644 CrossRef CAS PubMed.
- The randomness factor R is known to take values close to 0 in a block copolymer, close to 1 in a random one, and 1 < R ≤ 2 in alternating polymers: see ref. 20h.
- C. Bonné, A. Pahwa, C. Picard and M. Visseaux, Inorg. Chim. Acta, 2016, 455, 521 CrossRef.
- Y. T. Huang, W. C. Wang, C. P. Hsu, W. Y. Lu, W. J. Chuang, M. Y. Chiang, Y. C. Laia and H. Y. Chen, Polym. Chem., 2016, 7, 4367 RSC.
- D. Specklin, C. Fliedel, F. Hild, S. Mameri, L. Karmazin, C. Bailly and S. Dagorne, Dalton Trans., 2017, 46, 12824 RSC.
- F. Hild, N. Neehaul, F. Bier, M. Wirsum, C. Gourlaouen and S. Dagorne, Organometallics, 2013, 32, 587 CrossRef CAS.
- A. Harinath, J. Bhattacharjee, A. Sarkar, H. P. Nayek and T. K. Panda, Inorg. Chem., 2018, 57, 2503 CrossRef CAS PubMed.
- S. Liu, J. Zhang, W. Zuo, W. Zhang, W.-H. Sun, H. Ye and Z. Li, Polymers, 2017, 9, 83 CrossRef.
- Y. Nakayama, K. Sasaki, N. Watanabe, Z. Cai and T. Shiono, Polymer, 2009, 50, 4788 CrossRef CAS.
- T. J. J. Whitehorne and F. Schaper, Can. J. Chem., 2014, 92, 206 CrossRef CAS.
- B. J. Jeffery, E. L. Whitelaw, D. Garcia-Vivo, J. A. Stewart, M. F. Mahon, M. G. Davidson and M. D. Jones, Chem. Commun., 2011, 47, 12328 RSC.
- J. Fagerland, A. Finne-Wistranda and D. Pappalardo, New J. Chem., 2016, 40, 7671 RSC.
- F. M. García-Valle, V. Tabernero, T. Cuenca, M. E. G. Mosquera, J. Cano and S. Milione, Organometallics, 2018, 37, 837 CrossRef.
- E. Brule, V. Guerineau, P. Vermaut, F. Prima, J. Balogh, L. Maron, A. M. Z. Slawin, S. P. Nolan and C. M. Thomas, Polym. Chem., 2013, 4, 2414 RSC.
- J. Lewiński, P. Horeglad, K. Wójcik and I. Justyniak, Organometallics, 2005, 24, 4588 CrossRef.
- To account for the reversely higher reactivity of LA vs. ε-CL in statistical ROcoP processes using Al(OiPr)3, the rate constant of the PLA growing chain was 20 to 30 times higher for LA [PLA-O-Al + monomer LA ] than for CL [PLA-O-Al + monomer CL], whereas the rate constants of the PCL growing chain [PCL-O-Al + monomer (LA or CL)] were several orders of magnitude higher than those of PLA growing chain [PLA-O-Al + monomer (LA or CL)]. This means that there is a quasi-equal probability of LA or CL to be inserted into a growing PCL chain while as soon as a LA monomer is inserted, this will favour the insertion of a next LA rather than ε-CL. See ref. 17c.
- See for example: (a) C. Jacobs, P. Dubois, R. Jérôme and P. Teyssié, Macromolecules, 1991, 24, 3027 CrossRef CAS; (b) M. Florczak, J. Libiszowski, J. Mosnacek, A. Duda and S. Penczek, Macromol. Rapid Commun., 2007, 28, 1385 CrossRef CAS.
- D. Chandanbodhi and T. Nanok, Mol. Catal., 2017, 436, 145 CrossRef.
- A. Valente, P. Zinck, A. Mortreux and M. Visseaux, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1615 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |