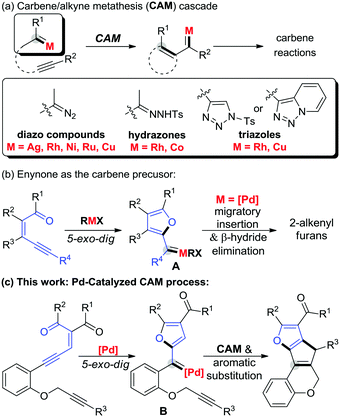
Palladium-catalyzed carbene/alkyne metathesis with enynones as carbene precursors: synthesis of fused polyheterocycles†
Yang
Zheng
a,
Ming
Bao
a,
Ruwei
Yao
a,
Lihua
Qiu
*a and
Xinfang
Xu
 *ab
*ab
aKey Laboratory of Organic Synthesis of Jiangsu Province; College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, P. R. China. E-mail: qiulihua@suda.edu.cn; xinfangxu@suda.edu.cn
bState Key Laboratory of Elemento-organic Chemistry, Nankai University, Tianjin 300071, China
First published on 4th December 2017
Abstract
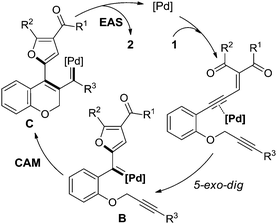
An unprecedented palladium-catalyzed novel carbene/alkyne metathesis cascade reaction of alkyne-tethered enynones is described. This reaction affords fused polyheterocycles in moderate to good yields. The transformation begins with Pd-catalyzed 5-exo-dig cyclization of the enynone to form the donor/donor metal carbene, which then undergoes metathesis with the alkyne followed by electrophilic aromatic substitution.
A carbene mediated cascade reaction is a powerful tool for building new bonds.1 In particular, transformations that involve carbene/alkyne metathesis (CAM) have shown high efficiency in bond formations, which leads to direct construction of complex molecular frameworks.2–6 The pioneering work in this area reported by Padwa involves alkyne-tethered diazo compounds, with silver2a and rhodium complexes2b,c as catalysts. Later, various metal catalysts, including nickel,3a,b ruthenium,3c–f and copper,4a,b were disclosed to promote the corresponding cascade transformations. On the other hand, the use of other readily available and relatively stable substrates as the initiator, such as, triazole or hydrazone, instead of diazo compounds, was developed by Gevorgyan,5 May,6a Pla-Quintanaa,6b,c and Zhang and Bruin6d independently (Scheme 1a). Despite the advances in these cascade reactions involving CAM, the substrate scope is still limited, and the development of a practicable carbene precursor with a compatible catalytic system would certainly be in demand.
As a reliable and useful non-diazo carbenoid precursor, enynones have been applied in a wide range of carbene reactions.7 And the key step in these transformations is a transition metal catalyzed 5-exo-dig cyclization to generate the donor/donor carbene intermediate (Scheme 1b, A).8a Once formed in situ, the metal carbene intermediate can undergo various subsequent reactions such as C–H and X–H insertion,8 cycloaddition/annulation,9 coupling reactions,10 and others,11 to provide the corresponding furan derivatives. Inspired by these works and as the continuation of our interest in polycyclic structure construction based on the CAM reaction,4 herein, we describe a novel carbene/alkyne metathesis cascade reaction with enynones as the carbene precursor. In comparison with the Pd-catalyzed general coupling reactions of enynones via a migratory insertion process reported by Wang (Scheme 1b),10a–c this work shows the first example of a readily available palladium complex catalyzed carbene/alkyne metathesis reaction (Scheme 1c). The 3,4-disubstituted 2H-chromene product generated via this cascade reaction is prevalent in natural products and bioactive molecules.12
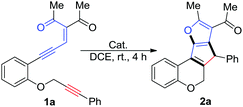
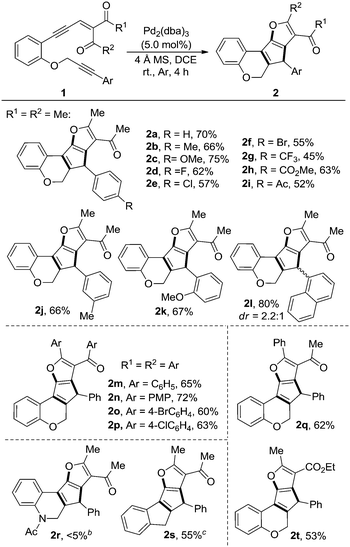
Alkyne-tethered enynone 1a was first prepared according to a known procedure (see the ESI† for details) and then utilized as a model substrate to probe the cascade reaction: it was mixed with various catalysts (with 1.0–5.0 mol%), including Rh, Au, Zn and Pd (Table 1, entries 1–12), in 1,2-dichloroethane (DCE). Except for PPh3AuCl, which exhibited no reactivity at all and majority of the material 1a was recovered (entry 4), most of the tested catalysts could promote the conversion of 1a, although low selectivities were observed in some cases and the rest of the material was decomposed into a complex mixture (entries 5–8). Lower reactivity was found with Pd(PPh3)4 (entry 11) compared to the Pd(II)-catalyst (entry 10). Using Pd2(dba)3 as the catalyst led to a significant improvement in the reaction and 2a was obtained in 62% isolated yield, which may due to the relatively low Lewis acidity (or π-electrophilicity) and weakly coordinating ligands of this catalyst (entry 12). Elevating the reaction temperature did not provide a better result (entry 13); however, introducing a 4 Å molecular sieve as an additive increased the yield to 70% (entry 14).
| Entry | Catalyst (x mol%) | Yieldb (%) |
|---|---|---|
| a The reaction was conducted with 1a (0.2 mmol), catalyst (x mol%) in DCE (3.0 mL) at 25 °C under Ar. b Isolated yields. c Most of 1a was recovered. d The reaction was conducted at 40 °C. e 4 Å molecular sieve (30 mg) was added as an additive. DCE = 1,2-dichloroethane, nr = no reaction. | ||
| 1 | Rh2(OAc)4 (1.0) | 20 |
| 2 | [Rh(COD)OH]2 (2.0) | 30 |
| 3 | Cu(OTf)2 (5.0) | 18 |
| 4 | PPh3AuCl (5.0) | nrc |
| 5 | PPh3AuCl (5.0) + AgSbF6 (5.0) | <5 |
| 6 | AgSbF6 (5.0) | <5 |
| 7 | PPh3AuCl (5.0) + AgBF4 (5.0) | <5 |
| 8 | AgBF4 (5.0) | <5 |
| 9 | ZnCl2 (20.0) | 25 |
| 10 | Pd(OAc)2 (5.0) | 37 |
| 11 | Pd(PPh3)4 (5.0) | 26 |
| 12 | Pd2(dba)3 (5.0) | 62 |
| 13d | Pd2(dba)3 (5.0) | 57 |
| 14e | Pd2(dba)3 (5.0) | 70 |
With the optimized reaction conditions in hand, we went on to explore the substrate scope of this reaction, and the results are listed in Table 2. The influence of the aryl group, which is adjacent to the alkyne moiety, was explored first (2a–l). Regardless of the position or electronic properties of the substitutions on the aryl ring, all the substrates gave the products in moderate to good yields (2a–2k). Interestingly, two diastereoisomers of the product 2l were obtained, which arises from the restriction of rotation between the two aromatic planes.13 Meanwhile, the reaction of the aryl-substituted enynones proceeded equally well, leading to the desired products 2m–p in 60–72% yields. For material 1r, which has an “N” linker instead of “O”, although the reaction is quite clean according to the proton NMR of the crude reaction mixture (see Fig. S1 in the ESI†), however, the product was decomposed into a complex mixture during the isolation using column chromatography. When 1s, which has a methylene linker, was applied, although no reaction occurred in the presence of either Pd(OAc)2 or Pd2(dba)3, we did observe the product when the reaction was catalyzed by Rh2(OAc)4, and the corresponding product 2s was obtained in 55% yield after 12 hours. In addition, these reaction conditions were also successfully applied to the unsymmetrical enynone Z-1q and mono-carbonyl substrate 1t, which gave 2q and 2t in 62% and 53% yields, respectively. The methyl-substituted alkyne (1u) and terminal alkyne (1v) could not provide the corresponding products and the materials were decomposed into a complex mixture slowly, which may be mainly due to the lower stability of the in situ generated carbenoid intermediate compared to the corresponding aryl stabilized ones.
| a Reactions are carried out on a 0.2 mmol scale with Pd2(dba)3 (5.0 mol%), 4 Å molecular sieve (30 mg) in DCE (3.0 mL) at room temperature under an Ar atmosphere, and the yields are given in isolated yields. b The product was decomposed into a complex mixture during the isolation using column chromatography. c Catalyzed by Rh2(OAc)4 (2.0 mol%) and running for 12 h. |
|---|
|
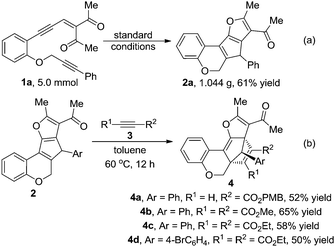
To demonstrate the scalability and the practicality of the current method, a gram scale reaction was carried out, and 1.044 g of 2a was isolated in 61% yield (Scheme 2a). [4+2] Cycloadditions applying the products from the cascade reaction were also investigated (Scheme 2b). Either terminal or internal electron deficient alkynes performed well under thermal conditions, and generated the corresponding addition products in moderate to good yields (4a–d). The structure of product 4d was unambiguously determined by single-crystal X-ray diffraction analysis,14 and the corresponding structure of 2 could be deduced.
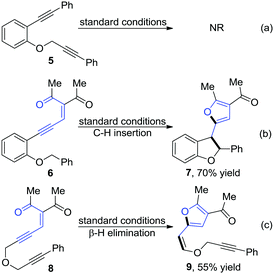
To gain insight into the mechanistic details, control experiments were carried out as shown in Scheme 3. Firstly, the enynone part in the material was removed, and no reaction occurred with 5 under the standard conditions (Scheme 3a), which means that the transformation was initiated by the enynone species. To demonstrate that the donor/donor carbenoid, which was generated from the enynone in situ, was involved in this transformation, two typical carbene reactions, including C–H insertion and β-H elimination, were designed and performed (Scheme 3b and c). Two corresponding products 7 and 9 were obtained in 70% and 55% yields, respectively. These results further confirmed the existence of the carbenoid intermediate B (Scheme 1c) in this reaction. Based on this information and previous works,2–4,15 we reasoned that the reaction was initiated by a palladium-catalyzed 5-exo-dig cyclization of the enynone to form the donor/donor carbenoid intermediate B, followed by carbene/alkyne metathesis (CAM), and terminated with electrophilic aromatic substitution (EAS) on the newly formed furan ring with the in situ generated carbenoid intermediate C (Scheme 4). However, further studies are needed to unambiguously establish the reaction transformation.
In summary, we have developed a palladium-catalyzed novel carbene/alkyne metathesis (CAM) cascade reaction of alkyne-tethered enynones, which provides a straightforward approach for the synthesis of furan fused polyheterocycles in moderate to high yields. In addition, this reaction not only represents the first example of a CAM reaction promoted by a readily available palladium complex, but also is the only example of the use of enynones as the carbene precursor in intramolecular carbene/alkyne metathesis cascade transformation. Potential applications of this strategy for polycyclic compounds synthesis are under exploration in our laboratory.
Support for this research from the National Natural Science Foundation of China and the NSFC of Jiangsu (NSFC21602148, BK20150315, and 15KJD150004), and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions is gratefully acknowledged.
Conflicts of interest
There are no conflicts to declare.Notes and references
- For review: (a) M. P. Doyle, R. Duffy, M. Ratnikov and L. Zhou, Chem. Rev., 2010, 110, 704 CrossRef CAS PubMed; (b) A. Padwa and M. D. Weingarten, Chem. Rev., 1996, 96, 223 CrossRef CAS PubMed; (c) H. M. L. Davies and Y. Lian, Acc. Chem. Res., 2012, 45, 923 CrossRef CAS PubMed; (d) Y. Xia, Y. Zhang and J. Wang, ACS Catal., 2013, 3, 2586 CrossRef CAS; (e) X. Guo and W. Hu, Acc. Chem. Res., 2013, 46, 2427 CrossRef CAS PubMed; (f) Q. Xiao, Y. Zhang and J. Wang, Acc. Chem. Res., 2013, 46, 236 CrossRef CAS PubMed; (g) A. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire and M. A. McKervey, Chem. Rev., 2015, 115, 9981 CrossRef CAS PubMed; (h) S. Jansone-Popova, P. Q. Le and J. A. May, Tetrahedron, 2014, 70, 4118 CrossRef CAS; (i) A. Caballero and P. J. Pérez, Chem. – Eur. J., 2017, 23, 14389 CrossRef CAS PubMed.
- Pioneering works: (a) A. Padwa, T. J. Blacklock and R. Loza, J. Am. Chem. Soc., 1981, 103, 2404 CrossRef CAS; (b) A. Padwa, D. J. Austin, Y. Gareau, J. M. Kassir and S. L. Xu, J. Am. Chem. Soc., 1993, 115, 2637 CrossRef CAS; (c) A. Padwa and M. D. Weingarten, J. Org. Chem., 2000, 65, 3722 CrossRef CAS PubMed; (d) A. Padwa and C. S. Straub, Org. Lett., 2000, 2, 2093 CrossRef CAS PubMed.
- Selected advances: (a) Y. Ni and J. Montgomery, J. Am. Chem. Soc., 2006, 128, 2609 CrossRef CAS PubMed; (b) Y. Ni and J. Montgomery, J. Am. Chem. Soc., 2004, 126, 11162 CrossRef CAS PubMed; (c) C. González-Rodríguez, J. R. Suárez, J. A. Varela and C. Saá, Angew. Chem., Int. Ed., 2015, 54, 2724 CrossRef PubMed; (d) D. Padín, F. Cambeiro, M. Fañanás-Mastral, J. A. Varela and C. Saá, ACS Catal., 2017, 7, 992 CrossRef; (e) F. Cambeiro, S. López, J. A. Varela and C. Saá, Angew. Chem., Int. Ed., 2014, 53, 5959 CrossRef CAS PubMed; (f) F. Cambeiro, S. López, J. A. Varela and C. Saá, Angew. Chem., Int. Ed., 2012, 51, 723 CrossRef CAS PubMed; (g) S. Jansone-Popova and J. A. May, J. Am. Chem. Soc., 2012, 134, 17877 CrossRef CAS PubMed; (h) Y. Qian, C. S. Shanahan and M. P. Doyle, Eur. J. Org. Chem., 2013, 6032 CrossRef CAS.
- Our works: (a) R. Yao, G. Rong, B. Yan, L. Qiu and X. Xu, ACS Catal., 2016, 6, 1024 CrossRef CAS; (b) C. Zhang, S. Chang, L. Qiu and X. Xu, Chem. Commun., 2016, 52, 12470 RSC; (c) Y. Zheng, J. Mao, Y. Weng, X. Zhang and X. Xu, Org. Lett., 2015, 17, 5638 CrossRef CAS PubMed; (d) X. Wang, Y. Zhou, L. Qiu, R. Yao, Y. Zheng, C. Zhang, X. Bao and X. Xu, Adv. Synth. Catal., 2016, 358, 1571 CrossRef CAS.
- With triazoles: (a) S. Chuprakov, F. W. Hwang and V. Gevorgyan, Angew. Chem., Int. Ed., 2007, 46, 4757 CrossRef CAS PubMed; (b) Y. Shi and V. Gevorgyan, Org. Lett., 2013, 15, 5394 CrossRef CAS PubMed; (c) Y. Shi and V. Gevorgyan, Chem. Commun., 2015, 51, 17166 RSC; (d) V. Helan, A. V. Gulevich and V. Gevorgyan, Chem. Sci., 2015, 6, 1928 RSC.
- With hydrazones: (a) P. Q. Le and J. A. May, J. Am. Chem. Soc., 2015, 137, 12219 CrossRef CAS PubMed; (b) Ó. Torres, T. Parella, M. Solà, A. Roglans and A. Pla-Quintana, Chem. – Eur. J., 2015, 21, 16240 CrossRef PubMed; (c) Ó. Torres, A. Roglans and A. Pla-Quintanaa, Adv. Synth. Catal., 2016, 358, 3512 CrossRef; (d) N. D. Paul, S. Mandal, M. Otte, X. Cui, X. P. Zhang and B. de Bruin, J. Am. Chem. Soc., 2014, 136, 1090 CrossRef CAS PubMed.
- For reviews, see: (a) J. Ma, L. Zhang and S. Zhu, Curr. Org. Chem., 2016, 20, 102 CrossRef CAS; (b) M. Jia and S. Ma, Angew. Chem., Int. Ed., 2016, 55, 9134 CrossRef CAS PubMed; (c) D. Qian and J. Zhang, Chem. Soc. Rev., 2015, 44, 677 RSC.
- C–H insertion: (a) K. N. Lamb, R. A. Squitieri, S. R. Chintala, A. J. Kwong, E. I. Balmond, C. Soldi, O. Dmitrenko, M. C. Reis, R. Chung, J. B. Addison, J. C. Fettinger, J. E. Hein, D. J. Tantillo, J. M. Fox and J. T. Shaw, Chem. – Eur. J., 2017, 23, 11843 CrossRef CAS PubMed; (b) D. Zhu, J. Ma, K. Luo, H. Fu, L. Zhang and S. Zhu, Angew. Chem., Int. Ed., 2016, 55, 8452 CrossRef CAS PubMed ; X–H insertion: ; (c) Y. Yu, S. Yi, C. Zhu, W. Hu, B. Gao, Y. Chen, W. Wu and H. Jiang, Org. Lett., 2016, 18, 400 CrossRef CAS PubMed; (d) J. González, J. González, C. Pérez-Calleja, L. A. López and R. Vicente, Angew. Chem., Int. Ed., 2013, 52, 5853 CrossRef PubMed; (e) S. Mata, L. A. López and R. Vicente, Chem. – Eur. J., 2015, 21, 8998 CrossRef CAS PubMed; (f) J.-M. Yang, Z.-Q. Li, M.-L. Li, Q. He, S.-F. Zhu and Q.-L. Zhou, J. Am. Chem. Soc., 2017, 139, 3784 CrossRef CAS PubMed.
- (a) R. Vicente, J. González, L. Riesgo, J. González and L. A. López, Angew. Chem., Int. Ed., 2012, 51, 8063 CrossRef CAS PubMed; (b) S. Mata, L. A. López and R. Vicente, Synlett, 2015, 2685 CAS; (c) S. Mata, J. González, L. A. López and R. Vicente, Eur. J. Org. Chem., 2016, 2681 CrossRef CAS; (d) M. J. González, L. A. López and R. Vicente, Org. Lett., 2014, 16, 5780 CrossRef PubMed; (e) M. J. González, E. López and R. Vicente, Chem. Commun., 2014, 50, 5379 RSC; (f) C. H. Oh, S. J. Lee, J. H. Lee and Y. J. Na, Chem. Commun., 2008, 5794 RSC; (g) J. Ma, H. Jiang and S. Zhu, Org. Lett., 2014, 16, 4472 CrossRef CAS PubMed; (h) H. Luo, K. Chen, H. Jiang and S. Zhu, Org. Lett., 2016, 18, 5208 CrossRef CAS PubMed; (i) S. Y. Hong, J. Jeong and S. Chang, Angew. Chem., Int. Ed., 2017, 56, 2408 CrossRef CAS PubMed; (j) B. Song, L.-H. Li, X.-R. Song, Y.-F. Qiu, M.-J. Zhong, P.-X. Zhou and Y.-M. Liang, Chem. – Eur. J., 2014, 20, 5910 CrossRef CAS PubMed.
- (a) Y. Xia, S. Qu, Q. Xiao, Z.-X. Wang, P. Qu, L. Chen, Z. Liu, L. Tian, Z. Huang, Y. Zhang and J. Wang, J. Am. Chem. Soc., 2013, 135, 13502 CrossRef CAS PubMed; (b) Y. Xia, R. Ge, L. Chen, Z. Liu, Q. Xiao, Y. Zhang and J. Wang, J. Org. Chem., 2015, 80, 7856 CrossRef CAS PubMed; (c) Y. Xia, Z. Liu, R. Ge, Q. Xiao, Y. Zhang and J. Wang, Chem. Commun., 2015, 51, 11233 RSC; (d) Y. Xia, L. Chen, P. Qu, G. Ji, S. Feng, Q. Xiao, Y. Zhang and J. Wang, J. Org. Chem., 2016, 81, 10484 CrossRef CAS PubMed; (e) F. Hu, Y. Xia, C. Ma, Y. Zhang and J. Wang, Org. Lett., 2014, 16, 4082 CrossRef CAS PubMed; (f) F. Hu, Y. Xia, C. Ma, Y. Zhang and J. Wang, J. Org. Chem., 2016, 81, 3275 CrossRef CAS PubMed.
- (a) Y. Kato, K. Miki, F. Nishino, K. Ohe and S. Uemura, Org. Lett., 2003, 5, 2619 CrossRef CAS PubMed; (b) T. Wang and J. Zhang, Dalton Trans., 2010, 39, 4270 RSC; (c) H. Cao, H. Zhan, J. Cen, J. Lin, Y. Lin, Q. Zhu, M. Fu and H. Jiang, Org. Lett., 2013, 15, 1080 CrossRef CAS PubMed; (d) H. Zhan, X. Lin, Y. Qiu, Z. Du, P. Li, Y. Li and H. Cao, Eur. J. Org. Chem., 2013, 2284 CrossRef CAS; (e) K. Miki, Y. Washitake, K. Ohe and S. Uemura, Angew. Chem., Int. Ed., 2004, 43, 1857 CrossRef CAS PubMed.
- (a) A. Arroio, E. F. Lima, K. M. Honório and A. B. F. da Silva, Struct. Chem., 2009, 20, 577 CrossRef CAS; (b) A. J. Walkinshaw, W. Xu, M. G. Suero and M. J. Gaunt, J. Am. Chem. Soc., 2013, 135, 12532 CrossRef CAS PubMed; (c) N. Stanković, M. Mladenović, M. Mihailović, J. Arambašić, A. Uskoković, V. Stanković, V. Mihailović, J. Katanić, S. Matić, S. Solujić, N. Vuković and S. Sukdolak, Eur. J. Pharm. Sci., 2014, 55, 20 CrossRef PubMed; (d) D. A. AlQudah, M. A. Zihlif and M. O. Taha, Eur. J. Med. Chem., 2016, 110, 204 CrossRef CAS PubMed.
- (a) S. Reichert and B. Breit, Org. Lett., 2007, 9, 899 CrossRef CAS PubMed; (b) M.-S. Seo, A. Lee and H. Kim, Org. Lett., 2014, 16, 2950 CrossRef CAS PubMed.
- CCDC 1545526 (4d)†.
- (a) A. S. K. Hashmi, Angew. Chem., Int. Ed. Engl., 1995, 34, 1581 CrossRef; (b) A. S. K. Hashmi, T. L. Ruppert, T. Knöfel and J. W. Bats, J. Org. Chem., 1997, 62, 7295 CrossRef CAS PubMed; (c) B. Alcaide, P. Almendros and T. M. Campo, Eur. J. Org. Chem., 2007, 2844 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1545526 (4d). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7cc08221e |
| This journal is © The Royal Society of Chemistry 2018 |