Palladium-catalyzed asymmetric allylic amination: enantioselective synthesis of chiral α-methylene substituted β-aminophosphonates†
Xubin
Wang
a,
Xiaoming
Wang
a,
Zhaobin
Han
a,
Zheng
Wang
*a and
Kuiling
Ding
*abc
aState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China. E-mail: kding@mail.sioc.ac.cn; Fax: +(21)-6416-6128
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
cCollaborative Innovation Center of Chemical Science and Engineering, Tianjin 300071, China
First published on 29th November 2016
Abstract
Spiroketal backbone based diphosphine ligands (SKP) were disclosed to be highly efficient and enantioselective (94 → 99% ee) in the palladium catalyzed asymmetric allylic amination of 2-diethylphosphonate-substituted allylic acetates, affording a series of chiral β-aminophosphonates bearing an α-methylene functionality in high yields with excellent regioselectivities.
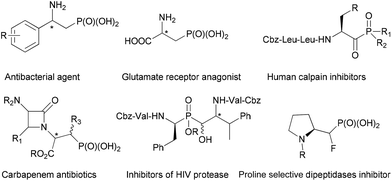
Amino phosphonic acids1 are phosphorus analogues of the corresponding amino acids, in which the planar and less bulky carboxylic acid group is replaced by a tetrahedral phosphonic acid functionality. In this context, β-amino phosphonic acids and their derivatives, as the isosteres of β-amino acids, have been revealed to possess a diverse range of medicinal properties including anti-bacterial,2 enzyme inhibitors,3 antibiotics,4 anti-HIV,5 and anti-inflammatory6 activities (Fig. 1). Given their growing importance in pharmaceutical applications, it is not surprising to see that the synthesis of β-amino phosphonic acid derivatives has attracted considerable interest of the synthetic community.7 Whereas a number of useful methods have been developed in general, there remains a dearth of efficient and versatile methodologies for the asymmetric synthesis of optically active β-amino phosphonic acid derivatives.8 Since the initial reports on the catalytic asymmetric synthesis of β-amino phosphonates via the amino hydroxylation of unsaturated phosphonates in the late 1990s,9 several types of catalytic systems have been documented to date, including the catalytic asymmetric hydrogenation of β-amido-vinylphosphonates,10 asymmetric Mannich reactions,11 and the desymmetrization of aziridines by phosphites.12 Alternatively, optically enriched β-amino phosphonates have also been obtained indirectly via catalytic asymmetric nitroaldol13 or phospha-Michael additions14 followed by reduction of the resulting β-nitroethylphosphonates. Despite these notable advances, catalytic stereoselective protocols that can provide an efficient direct access to chiral β-aminophosphonic acid derivatives are still scarce. Herein, we report a highly enantioselective catalytic asymmetric amination of 2-(diethylphosphonyl)-substituted allylic acetates, to afford a range of α-methylene-β-aminophosphonates in excellent optical purities.
Recently, our group has reported the development of spiroketal-based chiral diphosphine ligands (SKP),15 a new class of diphosphines with sterically well-defined spiro backbones.16 The SKP ligands were found to be highly efficient in the Pd catalyzed asymmetric allylic amination17 of racemic ethyl 2-(acetoxy(phenyl)methyl)acrylates, a type of Morita–Baylis–Hillman (MBH) adduct,18 to give the corresponding β-arylamino acid esters with high regio- and enantioselectivities.19 Kinetic and mechanistic studies indicated that the unusual long distance of the two P atoms in the SKP ligand allows for its unique role in the reaction, i.e. the ligand adopts an organo- and organometallic bifunctional mode in the cooperative catalysis.20 Encouraged by these results, we sought to extend the catalytic system to the asymmetric allylic amination of 2-(diethylphosphonyl)-substituted allylic acetates. The expected amination products would be enantioenriched β-aminophosphoric acid derivatives which can be viewed as the bioisosteres of α-methylene-β-amino acids that have very recently been found to be a key unnatural amino acid unit in a new class of endomorphin-1 analogues with potent antinociceptive activity.21 Furthermore, the olefin functionality present in the amination products may constitute a useful handle for further synthetic manipulation, thus giving ready access to a wider array of β-amino phosphoric acid derivatives.
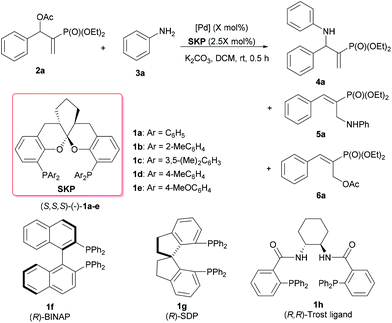
The study was initiated by a survey of the reaction conditions, including variations in palladium sources and SKP ligands, catalyst loadings, solvents, and bases, for the amination of 2-(diethylphosphonyl)-substituted allylic acetate (2a) with aniline (3a) as the nucleophile. The reactions were generally conducted at room temperature for 0.5 h, using the complex generated in situ from a SKP ligand [(S,S,S)-1a–e] and a palladium precursor as the catalyst. The effects of solvents and bases on the reaction of 2a and 3a were examined in the presence of the [Pd(η3-C3H5)Cl]2 (1.0 mol%)/(S,S,S)-1a (2.5 mol%) catalyst, indicating that both parameters have a significant impact on the reactivity as well as the chemo-, regio-, and enantioselectivities (for details, see Table S1 in the ESI†). In these cases, the reaction was found to be best performed in dichloromethane in the presence of two equivalents of anhydrous K2CO3 as the base, to afford the chiral allylic amination product 4a in 95% yield with excellent chemo-, regio-, and enantioselectivities (4a/5a/6a = >98/<2/0, >99% ee, entry 1 in Table 1). Under these optimized reaction conditions, the effects of catalyst compositions and loadings on the reaction of 2a and 3a were further evaluated, using SKP ligands (S,S,S)-1a–e with subtle variations in their aryl substituents at the P atoms and a couple of Pd precursors. The results are summarized in Table 1. With [Pd(η3-C3H5)Cl]2 (1.0 mol%) as the palladium precursor, a sharp difference in catalytic behavior was observed among the SKP ligands (S,S,S)-1a–e (entries 1–5). For example, high activity and excellent regio-/enantioselectivities were obtained using ligand 1a, 1c, or 1e with phenyl, 3,5-xylyl or 4-methoxyphenyl substituents, respectively, affording the targeted product 4a in high yields (92–95%) with 98–99% ee values (entries 1, 3, and 5). In contrast, ligand 1b possessing 2-tolyl moieties on the P atoms obviously deteriorates the reactivity and regioselectivity, leading to only very poor conversion (5%) and a modest branched/linear regioselectivity (4a/5a = 2/3) under otherwise identical conditions (entry 2). Intriguingly, 4-tolyl-bearing ligand 1d, with structural features analogous to both 1a and 1e, afforded much inferior chemoselectivity albeit with a 98% ee for 4a (entry 4), presumably as a result of incomplete amination of the isomerization product 6a within 0.5 h. Intriguingly, the reaction results with some privileged chiral ligands,16e.g., (R)-BINAP, (R)-SDP or (R,R)-Trost ligand, were less satisfactory under the otherwise identical conditions, affording incomplete conversions and moderate chemo-, regio- and enantioselectivities (entries 6–8). These facts clearly indicated that SKP ligands demonstrate unique performance in the catalysis of this type of asymmetric transformation. With 1a as the ligand, the use of different palladium precursors also resulted in distinct catalytic activities and selectivities (entries 1 and 9–11). While the use of Pd2(dba)3 delivers excellent results nearly identical to those of [Pd(η3-C3H5)Cl]2 (entries 9 vs. 1), Pd(OAc)2 or Pd(CH3CN)2Cl2 turns out to be much less efficient, realizing only partial conversion of 2a (entry 7) and lower yields of 4a (entries 10 and 11), or a substantial amount of the unreacted isomerization product 6a (entry 11). Further trials to lower the catalyst loadings were thus performed using either [Pd(η3-C3H5)Cl]2 or Pd2(dba)3 along with ligand 1a as the catalyst, and the reaction times were prolonged to 3 h (entries 12–16). Under these conditions, the loading of [Pd(η3-C3H5)Cl]2 was lowered to 0.5 mol% without loss of either yield of selectivities (entry 12), whereas further decreasing the loading to 0.1 mol% resulted in partial conversion and declined yield (entry 13). In this context, Pd2(dba)3 seems to be superior as the palladium precursor, and its loading can be lowered all the way to 0.1 mol% with essentially no changes in yields or ee values of 4a (entries 14 and 15 vs. 9). Further lowering of the Pd2(dba)3 loading to 0.05 mol%, however, led to a significant decrease in the reactivity albeit still with a 98% ee value for 4a (entry 16).
| Entry | [Pd]b (X mol%) | Ligandb | Conv.c (%) | 4a/5a/6ac | Yieldd (%) | eee (%) |
|---|---|---|---|---|---|---|
| a Unless otherwise noted, the reaction was performed with 2a (0.2 mmol) and 3a (0.4 mmol), K2CO3 (0.4 mmol) in CH2Cl2 (2 mL) at rt for 0.5 h. b The molar percent of the Pd salt relative to that of 2a. In each case, the loading of the SKP ligand was 1.25 equiv. relative to that of Pd. c Determined by 1H NMR spectroscopy. d Yield of the isolated 4a. e The ee value of 4a was determined by chiral HPLC. f The loading of 1a was 2.5 mol% relative to that of 2a. g The reactions were run for 3 h. | ||||||
| 1 | [Pd(C3H5)Cl]2 (1) | 1a | >99 | >98/<2/0 | 95 | >99 |
| 2 | [Pd(C3H5)Cl]2 (1) | 1b | 5 | 2/3/0 | — | — |
| 3 | [Pd(C3H5)Cl]2 (1) | 1c | >99 | 93/2/5 | 92 | >99 |
| 4 | [Pd(C3H5)Cl]2 (1) | 1d | >99 | 68/2/30 | 63 | 98 |
| 5 | [Pd(C3H5)Cl]2 (1) | 1e | >99 | >98/<2/0 | 94 | 98 |
| 6 | [Pd(C3H5)Cl]2 (1) | 1f | 34 | 31/69/0 | 9 | 43 |
| 7 | [Pd(C3H5)Cl]2 (1) | 1g | 16 | 70/30/0 | 10 | 4 |
| 8 | [Pd(C3H5)Cl]2 (1) | 1h | 57 | 56/34/10 | 28 | 10 |
| 9 | Pd2(dba)3 (1) | 1a | >99 | >98/<2/0 | 95 | 98 |
| 10f | Pd(OAc)2 (2) | 1a | 36 | 36/0/64 | 12 | 88 |
| 11f | Pd(CH3CN)2Cl2 (2) | 1a | >99 | 44/6/50 | 43 | 95 |
| 12g | [Pd(C3H5)Cl]2 (0.5) | 1a | >99 | >98/<2/0 | 95 | 98 |
| 13g | [Pd(C3H5)Cl]2 (0.1) | 1a | 87 | 89/0/11 | 82 | 97 |
| 14g | Pd2(dba)3 (0.2) | 1a | >99 | >98/<2/0 | 95 | 98 |
| 15g | Pd2(dba)3 (0.1) | 1a | >99 | >98/<2/0 | 94 | 98 |
| 16g | Pd2(dba)3 (0.05) | 1a | 64 | 94/0/6 | 57 | 98 |
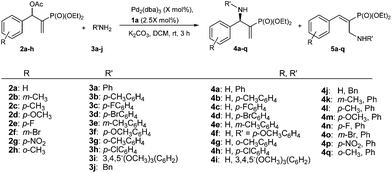
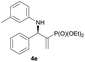
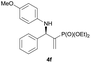
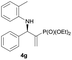
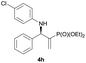
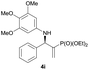
Subsequently, we proceeded to examine the substrate scope of the catalysis by variation in both the 2-diethylphosphonate-substituted allylic acetates (2a–h) and nucleophilic amines (3a–j). The reactions were run under the optimized conditions with a low loading of Pd2(dba)3 (0.1–0.5 mol%) and (S,S,S)-1a, and the results are summarized in Table 2. Gratifyingly, excellent enantioselectivities (94 → 99% ee) were observed in the resultant β-aminophosphonates 4a–q (entries 1–17). Both electron-donating and electron-withdrawing groups on the phenyl rings, located whether on the aromatic amine or on the allylic acetate, are well tolerated. The regioselectivities for the amination products (4/5) are also generally high, ranging from 90/10 to >98/2 (entries 1–16). The reaction involving substrate 2h was an exception (entry 17), however, giving a much higher content of the linear amination product (4q/5q = 61/39) and a moderate yield (40%) of 4q even at a relatively high loading of the catalyst (1.0 mol%), probably as a result of unfavorable interaction with the Pd catalyst caused by the sterically congested o-tolyl group in 2h. It is also noteworthy that the stereoelectronic properties of the aromatic amines have no obvious influence on the catalysis, as reactions of 2a with a range of anilines (3a–i) gave the corresponding products 4a–i in comparable good yields, high regioselectivities and excellent enantioselectivities (entries 1–9). The amination of 2a also proceeded smoothly with benzylamine 3j, an aliphatic nucleophile, to furnish β-aminophosphonate 4j in 84% yield with a 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
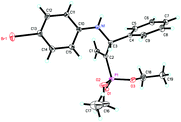
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 branched/linear ratio and 98% ee (entry 10). Finally, the absolute configuration of 4d was unambiguously established to be R by the X-ray crystal diffraction analysis (Fig. 2), while those for other products were deduced to be all R by comparison of their Cotton effects with that of (R)-(−)-4d as shown in the CD spectra (Fig. S2, ESI†).
10 branched/linear ratio and 98% ee (entry 10). Finally, the absolute configuration of 4d was unambiguously established to be R by the X-ray crystal diffraction analysis (Fig. 2), while those for other products were deduced to be all R by comparison of their Cotton effects with that of (R)-(−)-4d as shown in the CD spectra (Fig. S2, ESI†).
| Entry | 4 | X | 4/5b | Yieldc (%) | eed (%) |
|---|---|---|---|---|---|
| a Unless otherwise noted, the reactions were typically performed at rt with 2 (2.0 mmol) and 3 (4.0 mmol), K2CO3 (4.0 mmol) in CH2Cl2 (20 mL) for 3 h, in the presence of a specified amount of catalysts Pd2(dba)3 and (S,S,S)-1a. b Determined by 1H NMR spectroscopy. c Yield of the isolated 4a–q. d The ee values of 4a–q were determined by chiral HPLC. The absolute configurations for 4a–q were all determined to be R (see text). | |||||
| 1 |
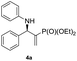
|
0.1 | >98/<2 | 94 | 98 |
| 2 |
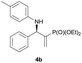
|
0.1 | 95/5 | 91 | 95 |
| 3 |
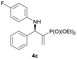
|
0.1 | >98/2 | 94 | 96 |
| 4 |
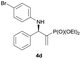
|
0.1 | 91/9 | 87 | 98 |
| 5 |
|
0.1 | 95/5 | 83 | 94 |
| 6 |
|
0.1 | 93/7 | 88 | 96 |
| 7 |
|
0.1 | 96/4 | 70 | 98 |
| 8 |
|
0.1 | 96/4 | 84 | 98 |
| 9 |
|
0.1 | >98/2 | 89 | 98 |
| 10 |
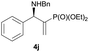
|
0.1 | 90/10 | 84 | 98 |
| 11 |
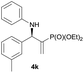
|
0.5 | 93/7 | 75 | 94 |
| 12 |
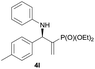
|
0.5 | 96/4 | 92 | 97 |
| 13 |
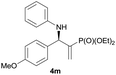
|
0.5 | 94/6 | 80 | 94 |
| 14 |
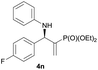
|
0.5 | 92/8 | 70 | 96 |
| 15 |
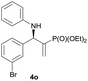
|
0.5 | 97/3 | 84 | 98 |
| 16 |
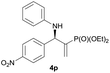
|
0.5 | 98/2 | 75 | >99 |
| 17 |
|
1 | 61/39 | 40 | 94 |
Conclusions
In conclusion, we have developed an efficient asymmetric synthesis of enantioenriched β-aminophosphonates bearing an α-methylene functionality. Using the complex generated in situ from the SKP ligand and a palladium precursor as the catalyst, asymmetric allylic amination of 2-diethylphosphonate-substituted allylic acetates proceeded smoothly under mild conditions with various amines as the nucleophiles, affording a series of β-aminophosphoric acid derivatives in good to excellent yields, high regioselectivities, and uniformly excellent enantioselectivities (94 → 99% ee). It is noteworthy that the olefin functionality present in the chiral β-aminophosphonate products may provide a useful handle for further synthetic manipulation, and thus may stimulate future explorations to use them as intermediates to access a wider array of β-amino phosphoric acid derivatives.Experimental
General procedures for SKP/Pd catalyzed asymmetric amination of 2-(diethylphosphonyl)-substituted allylic acetates
Into a Schlenk tube equipped with a magnetic stirring bar were added Pd2(dba)3 (1.8 mg, 0.005 mmol), (S,S,S)-1a (9.6 mg, 0.0125 mmol) and dichloromethane (5 mL) under a stream of argon. The solution was stirred for 5 min, followed by addition of 2 (0.5 mmol), K2CO3 (138 mg, 1.0 mmol) and 3 (1.0 mmol). The mixture was stirred for 3 h at room temperature, and then the solid residue was removed by filtration through a pad of Celite. The branched to linear ratio (4/5) of the amination products was determined by 1H NMR analysis of an aliquot of the filtrate. The solvent was removed in vacuo, and the residue was purified by flash chromatography on silica gel with petroleum ether/EA (1/2) as the eluent to afford optically enriched α-methylene β-amino phosphonate ester 4.The method can be applied in the Gram-scale preparation of β-aminophosphonates 4a under a reduced catalyst loading. By following the above mentioned procedure, the reaction of 2a (4.0 mmol, 1.24 g) with 3a (740 mg, 8.0 mmol) proceeded smoothly at rt for 8 h in dichloromethane (38 mL) in the presence of Pd2(dba)3 (7.3 mg, 0.008 mmol), (S,S,S)-1a (13.2 mg, 0.02 mmol), and K2CO3 (1.1 g, 8.0 mmol), to give branched amination product 4a (1.17 g, 85% yield) with 98% ee.
Acknowledgements
We are grateful for financial support from the Ministry of Science of Technology of China (2016YFA0202900), NSFC (21232009, 20421091), CAS (QYZDY-SSW-SLH012), and the Science and Technology Commission of Shanghai Municipality.Notes and references
- For reviews see: (a) V. P. Kukha and H. R. Hudson, Aminophosphonic and Amino-phosphinic Acids. Chemistry and Biological Activity, Wiley, Chichester, UK, 2000 Search PubMed; (b) A. Muccha, P. Kafarski and L. Berlicki, J. Med. Chem., 2011, 54, 5955 CrossRef PubMed; (c) M. Ordóñez, H. Rojas-Cabrera and C. Cativiela, Tetrahedron, 2009, 65, 17 CrossRef PubMed.
- (a) J. G. Allen, F. R. Arthenton, M. J. Hall, C. H. Hassall, S. W. Holmes, R. W. Lambert, L. J. Nisbet and P. S. Ringrose, Nature, 1978, 272, 56 CrossRef CAS PubMed; (b) L. Maier and P. J. Diel, Phosphorus, Sulfur Silicon Relat. Elem., 1995, 107, 245 CrossRef CAS; (c) L. Maier and P. J. Diel, Phosphorus, Sulfur Silicon Relat. Elem., 1996, 109, 341 CrossRef.
- (a) R. T. Wester, R. J. Chambers, M. D. Green and W. R. Murphy, Bioorg. Med. Chem. Lett., 1994, 4, 2005 CrossRef CAS; (b) D. V. Patel, K. Reilly-Gauvin, D. E. Ryono, C. A. Free, W. L. Rogers, S. A. Smith, J. M. Deforrest, R. S. Oehl and E. W. Petrillo, J. Med. Chem., 1995, 38, 4557 CrossRef CAS PubMed; (c) J. Zygmunt, R. Gancarz, B. Lejczak, P. Wieczorek and P. Kafarski, Bioorg. Med. Chem. Lett., 1996, 6, 2989 CrossRef CAS; (d) M. Tao, R. Bihovsky, G. J. Wells and J. P. Mallamo, J. Med. Chem., 1998, 41, 3912 CrossRef CAS PubMed; (e) P. van der Veken, K. Senten, I. Kertèsz, A. Haemers and K. Augustyns, Tetrahedron Lett., 2003, 44, 969 CrossRef CAS.
- (a) J. T. Whitteck, W. Ni, B. M. Griffin, A. C. Eliot, P. M. Thomas, N. L. Kelleher, W. W. Metcalf and W. A. van der Donk, Angew. Chem., Int. Ed., 2007, 46, 9089 CrossRef CAS PubMed; (b) G. H. Hakimelahi and A. A. Jarrahpour, Helv. Chim. Acta, 1989, 72, 1501 CrossRef CAS.
- B. Stowasser, K. H. Budt, L. J. Qi, A. Peyman and D. Ruppert, Tetrahedron Lett., 1992, 33, 6625 CrossRef CAS.
- A. A. A. Al Quntar, R. Gallily, G. Katzavian and M. Srebnik, Eur. J. Pharmacol., 2007, 556, 9 CrossRef PubMed.
- For elegant reviews, see: (a) F. Palacios, C. Alonso and J. M. de los Santos, Chem. Rev., 2005, 105, 899 CrossRef CAS PubMed; (b) F. Orsini, G. Sello and M. Sisti, Curr. Med. Chem., 2010, 17, 264 CrossRef CAS PubMed. For selected examples, see: (c) C. Yuan, S. Li, C. Li, S. Chen, W. Huang, G. Wang, C. Pan and Y. Zhang, Pure Appl. Chem., 1996, 68, 907 CrossRef CAS; (d) E. V. Grishkun and O. I. Kolodyzhnyi, Russ. J. Gen. Chem., 2009, 79, 2705 CrossRef CAS; (e) H. Park, C.-W. Cho and M. J. Krische, J. Org. Chem., 2006, 71, 7892 CrossRef CAS PubMed; (f) B. Das, C. R. Reddy, S. Nagendra and M. Lingaiah, Tetrahedron Lett., 2011, 52, 3496 CrossRef CAS; (g) C. Carzon, M. Attolini and M. Maffei, Synthesis, 2011, 3109 Search PubMed; (h) C. Alonso, M. González, M. Fuertes, G. Rubiales and J. M. Ezpeleta, J. Org. Chem., 2013, 78, 3858 CrossRef CAS PubMed; (i) C. Garzon, M. Attolini and M. Maffei, Eur. J. Org. Chem., 2013, 3653 CrossRef CAS; (j) C. Garzon, M. Attolini and M. Maffei, Tetrahedron Lett., 2010, 51, 3772 CrossRef CAS.
- For an excellent review, see: J. Ma, Chem. Soc. Rev., 2006, 35, 630 RSC.
- (a) G. Cravotto, G. B. Giovenzana, R. Pagliarin, G. Palmisano and M. Sisti, Tetrahedron: Asymmetry, 1998, 9, 745 CrossRef CAS; (b) A. A. Thomas and K. B. Sharpless, J. Org. Chem., 1999, 64, 8379 CrossRef CAS PubMed.
- (a) R. Kadyrov, J. Holz, B. Schäffner, O. Zayas, J. Almena and A. Börner, Tetrahedron: Asymmetry, 2008, 19, 1189 CrossRef CAS; (b) J. Zhang, Y. Li, Z. Wang and K. Ding, Angew. Chem., Int. Ed., 2011, 50, 11743 CrossRef CAS PubMed; (c) M. Á. Chávez, S. Vargas, A. Suárez, E. Álvarez and A. Pizzano, Adv. Synth. Catal., 2011, 353, 2775 CrossRef; (d) J.-H. Xie and Q.-L. Zhou, Acta Chim. Sin., 2012, 70, 1427 CrossRef CAS.
- (a) J. C. Wilt, M. Pink and J. N. Johnston, Chem. Commun., 2008, 4177 RSC; (b) H. Zhang, X. Wen, L. Gan and Y. Peng, Org. Lett., 2012, 14, 2126 CrossRef CAS PubMed; (c) A. Kjærsgaard and K. A. Jørgensen, Org. Biomol. Chem., 2005, 3, 804 RSC.
- M. Hayashi, N. Shiomi, Y. Funahashi and S. Nakamura, J. Am. Chem. Soc., 2012, 134, 19366 CrossRef CAS PubMed.
- T. Mandal, S. Samanta and C.-G. Zhao, Org. Lett., 2007, 9, 943 CrossRef CAS PubMed.
- (a) M. Terada, T. Ikehara and H. Ube, J. Am. Chem. Soc., 2007, 129, 14112 CrossRef CAS PubMed; (b) J. Wang, L. D. Heikkinen, H. Li, L. Zu, W. Jiang, H. Xie and W. Wang, Adv. Synth. Catal., 2007, 349, 1052 CrossRef CAS.
- (a) X. Wang, Z. Han, Z. Wang and K. Ding, Angew. Chem., Int. Ed., 2012, 51, 936 CrossRef CAS PubMed; (b) X. B. Wang, P. Guo, X. Wang, Z. Wang and K. Ding, Adv. Synth. Catal., 2013, 355, 2900 CrossRef CAS.
- For a review, see: (a) Privileged Chiral Ligands and Catalysts, ed. Q.-L. Zhou, Wiley-VCH, Weinheim, 2011 Search PubMed. Spiro backbones have been recognized as one of the privileged structures for the construction of chiral ligands, for reviews, see: (b) J.-H. Xie and Q.-L. Zhou, Acta Chim. Sin., 2014, 72, 778 CrossRef CAS; (c) G. B. Bajracharya, M. A. Arai, P. S. Koranne, T. Suzuki, S. Takizawa and H. Sasai, Bull. Chem. Soc. Jpn., 2009, 82, 285 CrossRef CAS; (d) K. Ding, Z. Han and Z. Wang, Chem. – Asian J., 2009, 4, 32 CrossRef CAS PubMed. For early examples, see: (e) A. S. C. Chan, W.-H. Hu, C.-C. Pai and C.-P. Lau, J. Am. Chem. Soc., 1997, 119, 9570 CrossRef CAS; (f) M. A. Arai, M. Kuraishi, T. Arai and H. Sasai, J. Am. Chem. Soc., 2001, 123, 2907 CrossRef CAS PubMed; (g) Y. Fu, J.-H. Xie, A.-G. Hu, H. Zhou, L.-X. Wang and Q.-L. Zhou, Chem. Commun., 2002, 480 RSC. For our recent examples, see: (h) Z. Han, Z. Wang, X. Zhang and K. Ding, Angew. Chem., Int. Ed., 2009, 48, 5345 CrossRef CAS PubMed; (i) J. Li, G. Chen, Z. Wang, R. Zhang, X. M. Zhang and K. Ding, Chem. Sci., 2011, 2, 1141 RSC; (j) X. Liu, Z. Han, Z. Wang and K. Ding, Angew. Chem., Int. Ed., 2014, 53, 1978 CrossRef CAS PubMed; (k) Z. B. Han, Z. Wang and K. Ding, Adv. Synth. Catal., 2011, 353, 1584 CrossRef CAS; (l) Y. Zhang, Z. Han, F. Li, K. Ding and A. Zhang, Chem. Commun., 2010, 46, 156 RSC; (m) J. Shang, Z. B. Han, Y. Li, Z. Wang and K. Ding, Chem. Commun., 2012, 48, 5172 RSC; (n) P. Zhang, Z. Han, Z. Wang and K. Ding, Angew. Chem., Int. Ed., 2013, 52, 11054 CrossRef CAS PubMed; (o) P. Zhang, J. Liu, Z. Wang and K. Ding, Chin. J. Catal., 2015, 36, 100–105 CrossRef CAS; (p) X. Liu, Z. Han, Z. Wang and K. Ding, Acta Chim. Sin., 2014, 72, 849 CrossRef CAS; (q) X. Liu, Z. Han, Z. Wang and K. Ding, Sci. China Chem., 2014, 57, 1073 CrossRef CAS; (r) X. Jia, Z. Wang, C. G. Xia and K. Ding, Catal. Sci. Technol., 2013, 3, 1901 RSC; (s) X. Jia, Z. Wang, C. G. Xia and K. Ding, Chem. – Eur. J., 2012, 18, 15288 CrossRef CAS PubMed; (t) J. Li, W. Pan, Z. Wang, X. Zhang and K. Ding, Adv. Synth. Catal., 2012, 354, 1980 CrossRef CAS.
- For reviews, see: (a) B. M. Trost, Acc. Chem. Res., 1996, 29, 355 CrossRef CAS; (b) B. M. Trost and D. L. Van Vranken, Chem. Rev., 1996, 96, 395 CrossRef CAS PubMed; (c) B. M. Trost and M. L. Crawley, Chem. Rev., 2003, 103, 2921 CrossRef CAS PubMed; (d) L.-X. Dai, T. Tu, S.-L. You, W.-P. Deng and X.-L. Hou, Acc. Chem. Res., 2003, 36, 659 CrossRef CAS PubMed; (e) L. A. Agrofoglio, I. Gillaizeau and Y. Saito, Chem. Rev., 2003, 103, 1875 CrossRef CAS PubMed; (f) B. M. Trost, M. R. Machacek and A. Aponick, Acc. Chem. Res., 2006, 39, 747 CrossRef CAS PubMed; (g) G. Helmchen, A. Dahnz, P. Dübon, M. Schelwies and R. Weinhofen, Chem. Commun., 2007, 675 RSC; (h) Z. Lu and S. Ma, Angew. Chem., Int. Ed., 2008, 47, 258 CrossRef CAS PubMed.
- (a) M. Shi, F.-J. Wang and M.-X. Zhao, The Chemistry of the MBH Reaction, RSC Publishing, London, 2011 Search PubMed; (b) Y. Wei and M. Shi, Acc. Chem. Res., 2010, 43, 1005 CrossRef CAS PubMed; (c) D. Basavaiah and G. Veeraraghavaiah, Chem. Soc. Rev., 2012, 41, 68 RSC; (d) D. Basavaiah, B. S. Reddy and S. S. Badsara, Chem. Rev., 2010, 110, 5447 CrossRef CAS PubMed; (e) Z. Qiao, Z. Shafiq, L. Liu, Z.-B. Yu, Q.-Y. Zheng, D. Wang and Y.-J. Chen, Angew. Chem., Int. Ed., 2010, 49, 7294 CrossRef CAS PubMed; (f) Y. Wang, L. Liu, D. Wang and Y.-J. Chen, Org. Biomol. Chem., 2012, 10, 6908 RSC; (g) F.-L. Hu and M. Shi, Org. Chem. Front., 2014, 1, 587 RSC; (h) G. Ma and M. P. Sibi, Org. Chem. Front., 2014, 1, 1152 RSC.
- (a) X. Wang, F. Meng, Y. Wang, Z. Han, Y.-J. Chen, L. Liu, Z. Wang and K. Ding, Angew. Chem., Int. Ed., 2012, 51, 9276 CrossRef CAS PubMed. For further applications of SKPs in asymmetric catalysis, see: (b) Z. Y. Cao, X. Wang, C. Tan, X.-L. Zhao, J. Zhou and K. Ding, J. Am. Chem. Soc., 2013, 135, 8197 CrossRef CAS PubMed; (c) X. Wang, X. Wang, Z. Han, Z. Wang and K. Ding, Angew. Chem., Int. Ed., 2016 DOI:10.1002/anie.201609332; (d) R. Cao, J. Zhang, H. Zhou, H. Yang and G. Jiang, Org. Biomol. Chem., 2016, 14, 2191 RSC; (e) J. Liu, Z. Han, X. Wang, Z. Wang and K. Ding, J. Am. Chem. Soc., 2015, 137, 15346 CrossRef CAS PubMed; (f) Y. Miyazaki, N. Ohta, K. Semba and Y. Nakao, J. Am. Chem. Soc., 2014, 136, 3732 CrossRef CAS PubMed; (g) X.-F. Wei, Y. Shimizu and M. Kanai, ACS Cent. Sci., 2016, 2, 21 CrossRef CAS PubMed.
- X. Wang, P. Guo, Z. Han, X. Wang, Z. Wang and K. Ding, J. Am. Chem. Soc., 2014, 136, 405 CrossRef CAS PubMed.
- (a) Y. Wang, Y. Xing, X. Liu, H. Ji, M. Kai, Z. Chen, J. Yu, D. Zhao, H. Ren and R. Wang, J. Med. Chem., 2012, 55, 6224 CrossRef CAS PubMed; (b) X. Liu, Y. Wang, Y. Xing, J. Yu, H. Ji, M. Kai, Z. Wang, D. Wang, Y. Zhang, D. Zhao and R. Wang, J. Med. Chem., 2013, 56, 3102 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1012761. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00597g |
| This journal is © the Partner Organisations 2017 |