Thermo- and photo-enhanced microtubule formation from Ru(bpy)32+-conjugated tubulin†
Kosuke
Okeyoshi
ab,
Ryuzo
Kawamura
a,
Ryo
Yoshida
b and
Yoshihito
Osada
*a
aRIKEN Advanced Science Institute, 2-1 Hirosawa, Wako-shi, Saitama 351-0198, Japan. E-mail: osadayoshi@riken.jp; Fax: +81 48 467 9300; Tel: +81 48 467 2816
bDepartment of Materials Engineering, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan
First published on 1st November 2013
Abstract
Ru(bpy)32+-conjugated tubulin is able to substantially enhance polymerization to form microtubules with increased rate at lower temperatures. Additionally, the polymerization is enhanced by photo-irradiation and the possible mechanism is discussed focusing on the photo-thermal energy conversion.
The kinetic control of self-assembly of molecules into functional materials is crucial for a variety of materials such as polymers, supramolecules, and proteins. Microtubules (MTs), a type of cytoskeletal filaments, are tubular molecular assemblies of α/β-tubulin heterodimers, which form rigid cylindrical filaments with a diameter of 25 nm and lengths of tens of micrometers. It is widely known that tubulin undergoes self-assembly when the temperature is raised to 37 °C to form geometrically ordered cylindrical MTs, which de-polymerize reversibly to form tubulin when the temperature is decreased. The dynamic equilibrium between MT self-assembly and disassembly has been well researched.1–5 The equilibrium state is attained when the growth of MTs due to self-assembly is exactly balanced by the disassembly of the MTs, which is connected to the hydrolysis of guanosine triphosphate (GTP) attached to the tubulin. In some cases, the rigid cylindrical filaments can be oriented in parallel bundles by integrating motor proteins6,7 or by an external impetus such as gravity,8 magnetic field,9 and temperature gradient.10 Ru(bpy)32+ is a well-known photosensitizer that absorbs photoenergy with a peak wavelength of around 460 nm.11–13 Since the aqueous solution of Ru(bpy)32+ in the excited state, *Ru(bpy)32+, shows a long lifetime of 650 ns, Ru(bpy)32+ has been widely considered as a photosensitizer for water splitting to yield O2 and protons that can combine with metal catalysts or organic pigments. In this work, we covalently introduced Ru(bpy)32+ onto α/β-tubulin by amine coupling14 and investigated the effect of the presence of Ru(bpy)32+-conjugated on tubulin on the equilibrium dynamics of the MT assembly (Fig. 1). The bulky structure and positive charges of the Ru(bpy)32+ molecule conjugated on the outer surface of tubulin can be expected to cause substantial changes to the self-assembly process of tubulin. In addition, photothermal effects may also be expected because *Ru(bpy)32+ releases a substantial portion of the energy (about 95%) by a non-radiative process.15 This phenomenon is very different from conventionally used fluorescent reagents such as rhodamine B (which radiate about 10% of the energy non-radiatively). In terms of such a photoenergy conversion, fluorescence reagent-bound tubulin should be useful for direct imaging of MTs and for the polymerization by using thermal energy in the non-radiative process. So far, several effects of photo-irradiation on microtubules have been studied, such as effects on stability16 and high-resolution imaging with a photoswitchable fluorescence reagent,17 but the effects on polymerization are still unclear. From our experiments, we found that the Ru(bpy)32+-conjugated tubulin can substantially enhance the rate of polymerization at lower temperatures, which was further increased by photo-irradiation. In this manuscript, we will discuss the possible mechanism of this phenomenon.
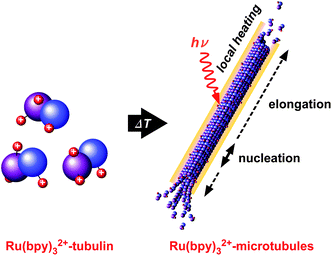 | ||
| Fig. 1 Schematic illustration of the formation of photo-enhanced MTs assembled from Ru(bpy)32+-conjugated tubulin. | ||
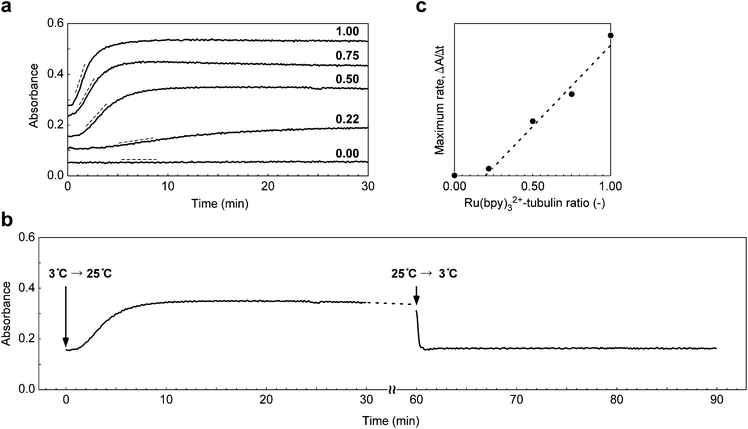
The self-assembly of tubulin was carried out by rapidly heating a solution of Ru(bpy)32+-conjugated tubulin and a non-conjugated tubulin mixture from 3 to 25 °C. The process was followed by measuring the change in the absorption at 350 nm.18Fig. 2a shows the chronology of the absorption changes that take place in the tubulin solution containing Ru(bpy)32+ at various ratios. It is seen that the non-conjugated tubulin showed no increase in absorption at 25 °C, indicating that tubulin did not form any MTs. However, when Ru(bpy)32+–tubulin was added, the absorption intensity increased with time. Further, the higher the concentrations of Ru(bpy)32+–tubulin was, the higher was the absorption. The self-assembly process seemed to be completed within 10 min when the Ru(bpy)32+–tubulin ratio was higher than 0.5. The increase in the base line was because of the absorption of Ru(bpy)32+. When the temperature was lowered from 25 °C to 3 °C (Fig. 2b), the absorption instantaneously decreased to the original absorption level, indicating that the Ru(bpy)32+-containing MTs depolymerized to yield a tubulin mixture. Hence, this self-assembly was a totally reversible process. Fig. 2c shows the apparent rate of assembly at 25 °C derived from the maximum slope in Fig. 2a as a function of the Ru(bpy)32+–tubulin ratio. The assembly occurred when the Ru(bpy)32+–tubulin ratio was more than 0.20 below which any assembly did not take place. Thus, a minimum Ru(bpy)32+–tubulin threshold concentration existed, which was required to promote the self-assembly at 25 °C. Besides, the rate of assembly increased linearly with an increase in the Ru(bpy)32+–tubulin ratio.
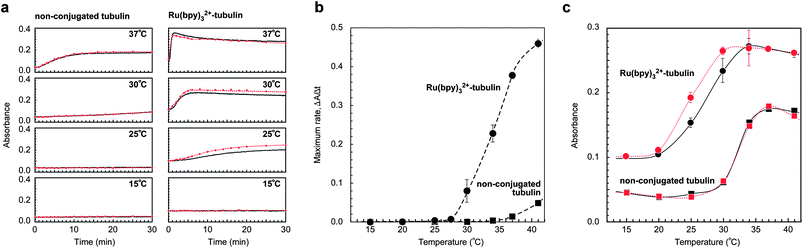
This phenomenon of the chemical attachment of Ru(bpy)32+ onto tubulin enhancing the self-assembly process is rather surprising because the bulky structure and the positive charges of Ru(bpy)32+ can be expected to interfere with the assembly process because of steric hindrance and the electrostatic repulsion. To understand the reason behind the increased rate of assembly with Ru(bpy)32+–tubulin at temperatures as low as 25 °C, the kinetics of the assembly was analyzed using a Ru(bpy)32+–tubulin ratio of 0.22 at temperatures ranging from 15 to 37 °C. As shown in Fig. 3a, non-conjugated tubulin did not increase the absorption, MT formation did not occur below 25 °C, and prominent absorption was observed at 37 °C. In contrast, as described before, a substantial increase in absorption was observed at 25 °C for Ru(bpy)32+-containing tubulin and the absorption rapidly increased with temperature. By plotting the maximum slope of the initial process in Fig. 3a as a function of temperature, the temperature dependence of the apparent rate of the assembly can be roughly obtained, as shown in Fig. 3b. In the case of the Ru(bpy)3–tubulin, the rate of assembly increased substantially with an increase in temperature, while the increase was slight in the case of the non-conjugated tubulin. At 37 °C, the rate was more than ∼30 times that shown by non-conjugated tubulin. The slope of Fig. 3b indicates the apparent overall activation energy of the process, and the overall activation energy of Ru(bpy)32+–tubulin was ∼15 times larger than that of the non-conjugated tubulin when the slopes between 34 °C and 37 °C were compared. A large difference in the activation energies of Ru(bpy)32+–tubulin and non-conjugated tubulin might be attributed to the positive charge on Ru(bpy)32+ that causes substantial hydration, which may critically affect the elementary process of the assembly.
The self-assembly of tubulin is a cooperative process and consists of nucleation and elongation.2,19,20 Bearing in mind that nucleation is the energy-consuming and the rate-determining step, the presence of Ru(bpy)32+ possibly enhances the nucleation through the substantial hydration. During nucleation, the short oligomer is formed. We could directly observe the preferential formation of oligomers in the Ru(bpy)32+–tubulin precursor by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), which suggests enhanced nucleation. As shown in Fig. S1a,† which shows the observation of the loaded gel with an optical band-pass filter (λ = 450 ± 25 nm), an illuminated band was observed only for the line of the Ru(bpy)32+-modified tubulin. This result clearly indicates that the Ru(bpy)32+ molecules were present in the band. Fig. S1b† shows the electrophoretic bands after the preparation of Ru(bpy)32+–tubulin by the amine coupling (after 1 h incubation at 37 °C and subjected to purification thrice). Some bands corresponding to oligomers of Ru(bpy)32+–tubulin such as the dimer (110 kDa), the trimer (165 kDa) could be observed, while only a single band corresponding to tubulin (55 kDa) was found. Short oligomers composed of a few tubulin subunits can assemble spontaneously; however, they are unstable and disassemble readily. At this stage, a GTP, which tightly binds to tubulin and catalytically enhances the polymerization by hydrolysis, dominates the overall polymerization process. When the GTP is hydrolyzed to yield guanosine diphosphate (GDP), it is known that much of the energy is released by the cleaving of the high-energy phosphate bonds. This energy is stored in the polymer lattice, making the free energy change of dissociation of the GDP-attached tubulin (which is responsible for the disassembly) higher than the free energy change of dissociation of the GTP-attached tubulin (which is responsible for the assembly). Consequently, the equilibrium constants of the dissociation of the GDP-attached tubulin and that of the GTP-attached tubulin are altered (causing dynamic instability). The enhanced assembly caused by conjugating Ru(bpy)32+ might be associated with the large difference in the activation energy, which could have led to the preferential formation of oligomers.
Based on the data obtained from the study of the kinetics (Fig. 3a), the transition curves for the tubulin/MTs (as a function of temperature) are summarized in Fig. 3c. The data were obtained by plotting the absorption strength observed at 20 min after the temperature change, when the apparent polymerization saturated. Interestingly, Fig. 3c (red curves) shows that photoirradiation of Ru(bpy)32+–tubulin additionally enhanced the MT formation at 25 °C and 30 °C, while enhancement of the polymerization was not observed at all for non-conjugated tubulin. The reason for such an enhancement is unclear; however, it can be speculated that the excited Ru(bpy)32+ released thermal energy non-radiatively i.e., *Ru(bpy)32+ → Ru(bpy)32+ + heat,15,21–23 leading to an increase in the local temperature around Ru(bpy)32+–tubulin. The thermal energy obtained by the conversion of the photoenergy by Ru(bpy)32+ can be estimated as Pxy = cmΔT, where P is the input photoenergy, x is the ratio of the absorbed photoenergy via the Ru(bpy)32+, y is the ratio of the non-radiative process, c is the specific heat of the sample solution, m is the mass of the sample solution, and ΔT is the temperature change in the sample solution per unit time. By calculating with values P < 88 mJ min−1, x < 1, y ∼ 0.95,15c ∼ 4.2 mJ mg−1 K−1,24m ∼ 12 mg, the converted thermal energy is capable of increasing the temperature as ΔT < 1.7 °C min−1. The sample used for the kinetics measurement was set in a holder with a thermostatic controller and the thermal energy converted from photoenergy should have had an effect mostly around the Ru(bpy)32+ molecules. Considering that microtubule depolymerization occurs at lower temperature than the polymerization (thermal hysteresis), the Ru(bpy)32+–microtubules apparently retain the formation when the thermostatic controller modulates a slight temperature change for isothermal conditions. Thus, Ru(bpy)32+–tubulin can behave as a photothermal energy sensitizer capable of promoting the assembly.
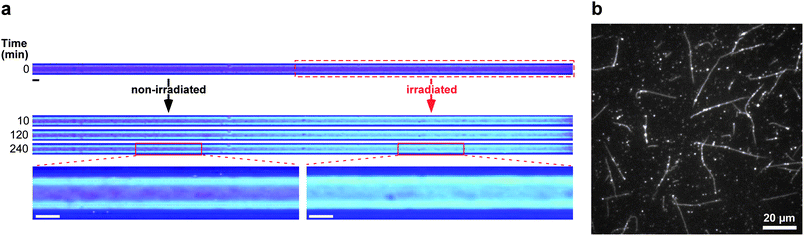
The effect of photothermal energy on the assembly process can be more clearly seen by observing the cross-polarized light using a cylindrical glass capillary. Fig. 4a shows the observation of the self-assembly from Ru(bpy)32+–tubulin after irradiating a part of the glass capillary at 29 °C.25 The formation of MTs can be observed by the changes in the color from dark violet to bright aqua blue after 10 min of irradiation. The figures indicate that the assembly of Ru(bpy)32+–tubulin was substantially enhanced by photo-irradiation, which began from the wall and proceeded to the central axis. The enhanced MT formation on the glass surface might be associated with the preferential adsorption of Ru(bpy)32+–tubulin to the negatively charged glass surface (see ESI, Fig. S2–S5†). Simultaneously, it seems that the Ru(bpy)32+–tubulin is capable of inducing the parallel orientation of microtubules in the glass capillary from the wall (see Fig. S4†). Further, fluorescence microscopy revealed entangled MT fibers with a length of 20–50 μm in the 5 μm gap between two cover glasses, as shown in Fig. 4b. The dotted bright spots randomly dispersed along the MT fibers could possibly be attributed to Ru(bpy)32+ and suggest that Ru(bpy)32+–tubulin has been randomly incorporated to the MTs. Thus, the covalently attached Ru(bpy)32+ substantially enhanced the self-assembly despite the sterically bulky structure and the positive charges. The study of the elementary process of Ru(bpy)32+–tubulin formation and the sequential structure of the obtained MT will be investigated in future.
Conclusions
In this work, we demonstrated the ability of Ru(bpy)32+-conjugated tubulin to form MTs by using thermal energy and photoenergy. Ru(bpy)32+–tubulin, unlike non-conjugated tubulin, underwent self-assembly to form MTs at lower temperatures as a result of an increase in the nucleation factors. In addition, by the non-radiative de-excitation of *Ru(bpy)32+ leading to the conversion of photoenergy into thermal energy, photo-enhancement of the MT formation was realized. Our findings suggest that the dynamics of the assembly can be controlled by integrating photoenergy, thermal energy, and electrostatic interactions. This strategy applied at the molecular level will shed light on the kinetic control of self-assembly of geometrical materials and will enable biological applications such as photo control of protein self-assembly.Experimental
Preparation of tubulin
To remove MT-associated proteins, tubulin was purified from porcine brain using high concentrations of PIPES buffer (1 M PIPES, 20 mM ethyleneglycol-bis(β-aminoethyl ether)-N,N,N0,N0-tetraacetic acid (EGTA), 10 mM MgCl2, pH adjusted to 6.8 using HCl).26 The tubulin concentration was determined by measuring the absorbance at 280 nm using an extinction coefficient of 115![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
Preparation of Ru(bpy)32+–tubulin
Ru(bpy)32+-conjugated tubulin was prepared using bis(2,2′-bipyridine)-4′-methyl-4-carboxybipyridine-ruthenium N-succinimidyl ester-bis(hexafluorophosphate) by amine coupling, according to a previously published method.14 The Ru(bpy)32+–tubulin stoichiometry, i.e., Ru(bpy)32+ molecules per tubulin, was estimated to be 3.1 ± 0.1/2.0 on average. The efficiency of labeling by the amine coupling was 31%. The concentration of Ru(bpy)32+ was determined by measuring the peak absorption strength at 460 nm (see ESI, Fig. S6 and S7†). The covalent conjugation of Ru(bpy)32+ on tubulin was confirmed by observing the loaded gel on an optical bandpass filter (λ = 450 ± 25 nm, BPB 45; Fujifilm). Ru(bpy)32+-conjugated MTs were obtained by polymerizing Ru(bpy)32+–tubulin and non-conjugated tubulin in BRB80 buffer (80 mM PIPES, 5 mM MgCl2, 1 mM EGTA, pH 6.8) with 5 mM guanosine-5′-triphosphate. Tubulin concentration in these mixtures was adjusted to 64 ± 5 μM, which was determined by the contrast of the stained band for SDS-PAGE.Measurement of MT self-assembly kinetics
Tubulin solutions in the optical cells (width = 1 mm, depth = 1 mm, and height = 45 mm) were maintained at 3 °C on ice and then placed in a thermostated holder in a spectrometer (UV-2500PC, Shimadzu) at various temperatures. Subsequently, one side of the optical cells was irradiated with visible light using a halogen lamp with a flat-surface light source (Techno Light KTS-100RSV, Kenko). The absorption strength was monitored at 350 nm at various times.Microscopic observation of the MTs
The Ru(bpy)32+–MTs were observed under a fluorescence microscope (IX71, Olympus) through a filter cube (AW078000 BP460–490 nm, LP663100 DM570 nm, AW098200 575 nm IF, U-MF2 Olympus) with an objective lens (Nikon, Plan Apo 100× oil/1.45) and an EM-CCD camera (Andor iXon+, AndorTechnology plc., Belfast, Northern Ireland). To observe the MT formation process, samples were photographed through linear cross polarizers with a wavelength retardation plate (460 ± 10 nm). See ESI, Fig. S2.†Acknowledgements
K. O. is grateful for the research fellowships of the Japan Society for the Promotion of Science for Young Scientists.Notes and references
- J. B. Olmsted and G. G. Borisy, Biochemistry, 1973, 12, 4282 CrossRef CAS.
- D. K. Fygenson, E. Braun and A. Libchaber, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 1994, 50, 1579 CrossRef CAS.
- J. Howard, Mechanics of Motor Proteins and the Cytoskeleton, Sinauer Associates, Sunderland, MA, 2001 Search PubMed.
- B. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts and P. Walter, Molecular Biology of the Cell, 4th edn, Garland Science, New York, 2002 Search PubMed.
- K. Sano, R. Kawamura, T. Tominaga, H. Nakagawa, N. Oda, K. Ijiro and Y. Osada, Biomacromolecules, 2011, 12, 1409 CrossRef CAS PubMed.
- R. Kawamura, A. Kakugo, Y. Osada and J. P. Gong, Langmuir, 2010, 26, 533 CrossRef CAS PubMed.
- T. Sanchez, D. Welch, D. Nicastro and Z. Dogic, Science, 2011, 333, 456 CrossRef CAS PubMed.
- J. Tabony and D. Job, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 6948 CrossRef CAS.
- Y. Liu, Y. Guo, J. M. Valles, Jr and J. X. Tang, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10654 CrossRef CAS PubMed.
- A. Kakugo, Y. Tamura, K. Shikinaka, M. Yoshida, R. Kawamura, H. Furukawa, Y. Osada and J. P. Gong, J. Am. Chem. Soc., 2009, 131, 18089 CrossRef CAS PubMed.
- K. Kalyanasundaram, Coord. Chem. Rev., 1982, 46, 159 CrossRef CAS.
- A. Yoshimura, M. Z. Hoffman and H. Sun, J. Photochem. Photobiol., A, 1993, 29 CrossRef CAS.
- J. H. Alsterum-Acevedo, M. K. Brennaman and T. J. Meyer, Inorg. Chem., 2005, 44, 6802 CrossRef PubMed.
- J. Peloquin, Y. Komarova and G. Borisy, Nat. Methods, 2005, 2, 299 CrossRef CAS PubMed.
- J. V. Caspar and T. J. Meyer, J. Am. Chem. Soc., 1983, 105, 5583 CrossRef CAS.
- G. P. A. Vigers, M. Coue and J. R. McIntosh, J. Cell Biol., 1988, 107, 1011 CrossRef CAS.
- M. Bates, B. Huang, G. T. Dempsey and X. Zhuang, Science, 2007, 317, 1749 CrossRef CAS PubMed.
- J. Tabony, Science, 1994, 264, 245 CAS.
- H. P. Erickson and D. Stoffler, J. Cell Biol., 1995, 135, 5 CrossRef.
- A. Desai and T. J. Mitchson, Annu. Rev. Cell Dev. Biol., 1997, 13, 83 CrossRef CAS PubMed.
- J. Van Houten and R. J. Watts, J. Am. Chem. Soc., 1976, 98, 4853 CrossRef CAS.
- M. Sykora, K. A. Maxwell, J. M. DeSimone and T. J. Meyer, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 7687 CrossRef CAS.
- B. Happ, A. Winter, M. D. Hager and U. S. Schubert, Chem. Soc. Rev., 2012, 41, 2222 RSC.
- The value is estimated for the overall average of the solution.
- The light intensity observed was sufficiently small; therefore, these experiments were considered as non-irradiated samples.
- M. Castoldi and A. V. Popov, Protein Expression Purif., 2003, 32, 83 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation of Ru(bpy)32+-conjugated tubulin, observation of MT formation under cross-polarized light, comments on MT formation and experimental details. See DOI: 10.1039/c3tb21242d |
| This journal is © The Royal Society of Chemistry 2014 |