Polymers with redox properties: materials for batteries, biosensors and more
Raquel Graciaa and David Mecerreyes*ab
aPOLYMAT, University of the Basque Country (UPV/EHU), Joxe Mari Korta Center, Avda. Tolosa 72, 20018 Donostia-San Sebastian, Spain
bIkerbasque, Basque Foundation for Science, E-48011 Bilbao, Spain. E-mail: david.mecerreyes@ehu.es
First published on 17th January 2013
Abstract
This minireview highlights the recent advances in the chemistry, characterization and applications of polymers with redox properties. The development of new redox polymers is clearly dominated by the interest in the area of batteries and biosensors. However, new applications in energy, materials science and biomedical fields have emerged together with the development of new polymeric materials. Historical works in the areas of ferrocene containing polymers and polyaniline conducting polymers have evolved today in a high number of innovative macromolecular structures whose singular properties indicate a bright future. The goal of this manuscript is to illustrate the state-of-the art in the development of polymers with redox properties and to highlight the most popular applications.
Introduction
Polymers with redox properties are those with the ability of changing their electrochemical properties with the oxidation state due to the loss of electrons (oxidation) or the gain of electrons (reduction). The IUPAC definition for a redox polymer is a polymer containing groups that can be reversibly reduced or oxidized. Reversible redox reaction can take place in the polymer main-chain, as in the case of conducting polymers such as polyaniline, or in side-groups, as in the case of a polymer carrying ferrocene side-groups. These properties depend on the nature of the polymer backbone (conjugated or non-conjugated) and the presence (or absence) of spatially localized redox groups. If the polymers present more than one redox site and/or a conjugated backbone the charge transfer may occur via electron hopping between the different redox centers or the semiconjugated backbone resulting in a complex multiple redox process.The redox process may be associated with changes in the properties of the polymeric material. Thus, depending on their oxidation state (oxidized or reduced) polymers can present different electronic properties such as ionic and electrical conductivity, optical properties, mechanical or chemical properties. Due to the reversibility and easy external control of the redox process, these polymers are interesting for different applications and the design of a number of electrochemical devices such as batteries, biosensors, electrochromic devices or biofuel cells. Furthermore, these polymers are finding new applications in materials science including the development of new types of actuators and drug delivery systems. Fig. 1 illustrates the examples of redox polymers and the most investigated application areas.
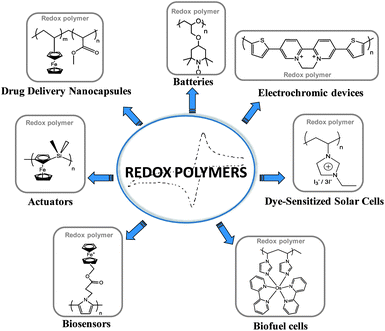 | ||
| Fig. 1 Chemical structures of the most popular applications and redox polymers. | ||
This article highlights the trends in redox polymer research. Firstly, the most recent synthetic and characterization advances will be reviewed followed by a description of the most popular applications and the polymers that are being investigated. The goal of this minireview is to provide an overview of the recent developments of polymers with redox properties including bibliographic references which the readers can follow to learn more about each particular polymer or application.
Synthesis of new polymers with redox properties
The first generation of redox polymers dates from the early 1980s when it was realized that the chemical modification of electrode surfaces would open up important new areas of science and technology. Over the years, redox polymers have incorporated a broad variety of chemical structures ranging from conjugated polymer backbones and electroactive organic or inorganic/organometallic moieties which have been included in the polymer backbone, as pendant side groups or both. Accordingly, different classifications have been proposed over the years which categorize the redox polymers depending on whether the redox center is in the polymer backbone or as a pendant group, the organic or inorganic nature of the redox center, or whether the polymers were electrically conducting polymers or more simple non-conductive redox polymers. These classifications always included hybrid forms and exceptions due to the complexity and the large variety of polymers that have been synthesized. For the sake of simplicity in this mini-review we will discuss the difference between polymers having fully organic redox centers and polymers having organometallic or partly inorganic redox centers. In both cases we will see examples where the redox center is in the polymer backbone or as a pendant side group or both. Our goal is to present a simple classification which allows us to review in an ordered way the most recent advances in each family of redox polymer.Redox polymers with electroactive organic redox couples
The development of redox polymers presenting organic chemical groups with redox properties such as nitroxyl, phenoxyl, carbazol, quinones, viologens, carbazol or hydrazyl is a very active research topic. We have selected some recent examples of polymers containing nitroxyl groups to initiate this section due to their technological interest. In a pioneering work, Nishide et al. reported a variety of redox polymers developed over the years containing stable nitroxide radicals such as TEMPO moieties. As it will be discussed in the Applications section these nitroxyl polymers are suitable active materials for organic batteries and supercapacitors (Fig. 2, polymer 1).1 Recent examples of new nitroxyl containing polymers include TEMPO–thiophene hybrid polymers which were developed in an attempt to find a synergy between the redox properties of the polythiophene backbone and the stable TEMPO moiety (polymer 2).2 Recently, Gohy et al. reported the development of new block copolymers which included the TEMPO redox moiety in one of their blocks (polymer 3).3 Future developments including new types of nitroxyl groups, hybrid materials combining nitroxides and other redox groups, block copolymers and new polymer architectures are currently under investigation. | ||
| Fig. 2 Chemical structures of polymers with organic redox moieties. | ||
Another important chemical group with redox properties that it is actively being incorporated into polymer materials is the carbonyl group.4 Different polymers including quinone, anthraquinone, quinine, anhydride or imide moieties are being investigated due to their redox ability. Examples of recent works in this area include the development of polymers such as the anthraquinone derivative (polymer 4), or the study of the redox properties of classic polymers such as polyimides (polymer 5).5,6 Interestingly, carbonyl compounds are widely available in natural cellulosic materials. Due to this, the area of hybrid materials between carbonyl containing natural polymers, such as lignin, and synthetic redox polymers is being investigated leading to materials with very interesting redox properties.
Other organic molecules, such as carbazole units, have been also incorporated into the polymer backbone in order to improve their electronic and optoelectronic properties in the preparation of thin films.7 Poly(vinylcarbazol) (PVCz) has been one of the most investigated polymers in this field as well as numerous soluble poly(carbazolyldiacetylenes).8Polymer 6 represents one example of this family.9 Polymers presenting phenazine organic molecules such as polymer 7 are also included in this group and the electrochemical preparation of other derivatives such as polyoxyphenazine is being investigated.10
The last group of redox couples incorporated into polymers of this family includes the organosulfur compounds. Different groups such as disulfides or polysulfides have been historically included into a variety of conjugated and non-conjugated polymeric backbones. Examples of recently developed disulfide–polysulfide polymers are polymers 8 and 9.11,12 Other important molecules incorporated into polymer backbones in order to improve the electronic and mechanical properties are the derivatives of tetrathiafulvalene (TTF).13,14 The ability of TTF to oxidize into cationic species (TTF+ and TTF2+) has been of interest in order to design functional polymers with redox properties such as polymer 10.
Redox polymers with electroactive inorganic redox couples
Ferrocene has been the most common organometallic redox moiety incorporated into different organic polymer backbones, including polymeric micro-nanoparticles or block copolymers, i.e. poly(vinyl ferrocene). In a recent example, a new ferrocene-containing epoxide monomer has been prepared in order to study its homo and copolymerization with ethylene oxide leading to water soluble polymers (see Fig. 3, polymer 11).15 Moreover, the ferrocene group has been incorporated into the shell of polymeric micro-nanoparticles. This was carried out in the form of polymer brushes synthesized by atom transfer polymerization of ferrocene functional methacrylate monomers.16 Alternatively, ferrocene has been incorporated into polymer backbones and a large number of poly(ferrocenylsilane) polymers were reported mainly by the groups of Vancso and Manners.17,18 These inorganic–organic polymers present very interesting stimuli responsive properties. Current works include the development of water soluble cationic polyferrocenyl silane polymers (polymer 12).19–21 Although ferrocene has extensively been incorporated into polymers the cobaltocene derivatives have been much less explored. As an example of the latter, the synthesis of cobaltocenium-containing block copolymers has been recently reported as a template for preparation of inorganic nanoparticles (polymer 13).22 | ||
| Fig. 3 Chemical structures of polymers with inorganic/organometallic redox moieties. | ||
Another family of polymers includes classic conjugated polymer structures where one of the atoms is substituted by an inorganic compound such as selenium, tellurium or phosphorus.23 Thus, recently, Heeney et al. reported the synthesis of poly(3-alkyl-2,5-selenylenevinylene) by a Still-cross coupling reaction in the presence of Pd(0) catalyst. Electron transporting studies on these selenophene-based polymers demonstrated their potential application in photovoltaic devices.24 Moreover, the propylenedioxyselenophene derivative (polymer 14) showed interesting electro-optical properties when the film is electrochemically doped.25 In the last few decades the groups of Chujo and Reau have been working on the development of new π-conjugated polymers containing phosphole units, as they showed interesting electron-donating properties in cyclic voltammetry studies.26,27 Moreover, other π-conjugated polymers incorporating phosphole and thiophene rings such as polymer 15 have also been reported, as they show excellent electro-optical properties.27 Lastly, it is worth noting that the recent development of poly(3-alkyltellurophene)s may open up a wide range of future studies involving tellurium-based polyheterocycles.23
The study of polymers containing transition metals has been rapidly expanded due to the well-known intrinsic properties of the metals such as catalytic, magnetic, redox, light absorption and emission.28 However, the electronic and electrochemical properties of the polymers containing transition metal atoms do not depend only on the properties of the metal unit incorporated as they will vary depending on the conjugation of the polymer main chain.29 As an example, when the metal compound is bonded to a conjugated polymer backbone the metal center could interact with the polymer chain involving changes in the optical properties such as absorption and emission spectra.30 In the case of a metal complex incorporated into a non-conjugated polymer the interaction may not occur and the metal center and polymer will emit and absorb separately. Based on these observations, a broad range of Zn(II) terpyridine based polymers presenting conjugated spacer groups, such as polymer 16, are being reported which show a tuned optical and electrochemical behavior. For these reasons, nowadays numerous transition metals are being integrated into polymers and as one recent example, ions such as Cu+ have been incorporated into polymer superstructures to achieve a double-helical formation with interesting electrical and optical properties.31
The last but not less important family of inorganic compounds includes the numerous osmium redox polymers synthesized in the last few years. Osmium redox polymers have mainly been investigated in the area of electrocatalysis and biosensing. For instance, Huang and coworkers have recently incorporated cellulose graft (HPC) osmium bipyrine into platinized carbon electrodes (polymer 17).32
Characterization and factors that affect the redox properties
Mainly electrochemical techniques have been applied for the characterization of redox polymers. Cyclic voltammetry, impedance spectroscopy or chronocoulometry are commonly used for the study of the redox polymer modified electrodes. Moreover, coupling of electrochemistry with other spectroscopic techniques such as UV-Vis, photo-emission, quartz microbalance and UV-NIR spectroscopy is useful in order to elucidate the changes of electronic, chemical and photophysical properties during the redox process.As a classic example, the commonly known conducting polymers (such as polypyrrole, polythiophenes, polyanilines and their derivatives) are macromolecules with a conjugated backbone whose electrochemical properties vary with the oxidation state.33 Cyclic voltammograms of conducting polymers show mixed phenomena between electron and ionic transfer during oxidation and reduction processes (non-Nernstian waves, Fig. 4a). On the other hand, polymers can incorporate redox active groups such as ferrocene into the polymer backbone or into pendant groups. The redox properties of non-conjugated polymers with only one redox site are only dependent on the properties of the redox center (Fig. 4b). Therefore, electrochemical properties of these types of polymers (oxidation or reduction) will be directly related to the properties of the redox system incorporated into the polymer chain (i.e. ferrocene/ferrocenium around +0.5 V vs. SCE). If the polymers present more than one redox site and/or a conjugated backbone the charge transfer may occur via electron hopping between the different redox centers or the semiconjugated backbone resulting in a complex multiple redox process and/or a rapid charge transfer through the delocalized chain (Fig. 4c).
 | ||
| Fig. 4 Representative examples of different redox processes in polymers: (a) typical cyclic voltammogram of conducting polymers (non-Nernstian waves), (b) non-conjugated redox polymers with one redox site such as ferrocene, and (c) example of polymers showing multiple redox processes. | ||
There are numerous factors that affect the redox properties of the polymers. Some of these factors are intrinsic to the polymer's chemical structure such as (i) the nature and localization of the redox center and (ii) conjugation of the backbone or between redox centers and (iii) ionic and conductivity properties of the redox polymer. For instance, during the redox process, polymers with conjugated backbone allow the charge transfer through the delocalized chain. This fact may result in an electronic communication between redox centers and a shift of the redox potential expected for the isolated redox sites.34 Electronic communication between redox centers could also happen via electron hopping between the redox moieties and this effect can be also followed by cyclic voltammetry studies. As an example, poly(ferrocenylsilane)s present two different reversible oxidation waves for the ferrocene units, as the ferrocene molecules in the polymer are electronically affected by the oxidation of the adjacent ferrocene system.35
On the other hand, redox properties of the polymers are affected by external factors associated with the measurement experiments such as (i) film thickness, (ii) kinetic processes associated with the slower electronic oxidation–reduction process of polymers, (iii) formulation of the electrode when conductive graphites and binders are added, (iv) nature of electrolyte, and (v) type of electrochemical cell and the working and reference counter-electrodes. These external factors justify different behaviours reported in the literature between similar polymers. The measurements should be also optimized depending on the application in which the redox polymers are going to be involved. For instance, in the preparation of modified electrodes for batteries or biosensor applications there are numerous issues that should be taken into account, such as electric conduction in the electrode, capability of charge transfer at the electrode–polymer interface, diffusion towards the different electrolytes or optimal contents of the active material in the electrode.36,37
Applications
The development of redox polymers has been without doubts an application oriented field. Historically, the most important applications have been (and still are) batteries and (bio)sensors. However, over the last few years redox polymers have been playing a crucial role in the development of other energy devices such as supercapacitors, solar cells and more recently promising biofuel cell technologies. Furthermore, recent synthetic advances are opening new opportunities in areas such as health with the development of drug delivery systems, actuators, catalysis or electronics.Electrochemical energy storage: batteries and supercapacitors
In order to provide the power for our modern and future life, researchers have made a lot of efforts to find new materials to build better batteries. The requirements for electrode materials have been focused mainly on the energy density, power density, charging–discharging rate, cyclability and safety. However, these will not be enough in the future if all the cars become electric-driven and rely on the inorganic oxides LiCoO2 and LiMn2O4 obtained from limited mineral resources. Although toxicity, safety and resource availability are known problems with lithium transition-metal oxides, alternatives have been hardly reported. Redox polymers which can be eventually synthesized from biomass, cost-effective and easily recycled seem to be a promising choice.Early attempts (80's and 90's) to incorporate redox polymers into batteries (cathodes or anodes) revolved around conjugated polymers such as polyaniline and polypyrrole.1 In a parallel way, polymers containing organosulfur moieties attracted great attention due to the ability to charge and discharge chemical groups such as disulfide in lithium batteries.38 However, electrodes manufactured from those polymers tended to have low specific energies, were sensitive to manufacturing, showed self-discharging drawbacks and experienced batch-to-batch variations which stopped the early excitement in the area. More recently in the 90–00's, the development of stable and commercially available conducting polymers and new organosulfur polymers such as poly(3,4-ethylenedioxy)thiophene (PEDOT) has launched new opportunities in the area.39,40
Early in this century, it was discovered that polymers bearing stable radicals such as TEMPO could be used as the electrode material in rechargeable batteries (Fig. 5). In the last decade, Nishide et al. developed a whole new set of radical polymers which increase the capability and cycling stability of organic radical batteries.41 An interesting and complete review in these developments has been very recently published by Schubert and coworkers and is recommended for more in-depth information and analysis about the behavior and performance of organic radical batteries.42 Interestingly, the electrochemical properties of radical polymers could be tuned in order to take part in electrodes for cathodes as well as anodes for batteries and mainly supercapacitors which seem to be in a commercialization process by the Japanese company NEC.
 | ||
| Fig. 5 Scheme of nitroxide organic radical/lithium metal batteries. | ||
In another pioneering work, Tarascon et al. demonstrated the promising charge–discharge capacities of some small organic molecules obtained from biomass (carbonyl compounds and conjugated lithium carboxylates).43 This work has opened a new research trend in the development of carbonyl containing polymers including quinone, anthraquinone, quinine, anhydride or imide moieties.44 Interestingly, conventional polyimides and polysulfones have been recently proposed as promising energy-storage materials.7
Fig. 6 summarizes the historical discoveries in the use of polymers in battery electrodes. These discoveries together with the potential of the macromolecular engineering open new opportunities in order to design tailor made redox polymers.
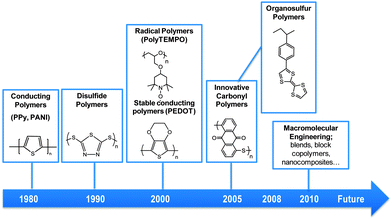 | ||
| Fig. 6 Timeline of the most important discoveries in polymers for batteries. | ||
New nanocomposites including carbon nanotubes or graphene, networks,45 blends and block copolymers are expected in the near future. The synergic combination of different redox polymers and/or the electrolytes in the same macromolecular assembly should surpass the performance of redox polymer electrodes. A seminal example of this was recently proposed by Inganäs et al. by designing an interpenetrating polymer network between a known conducting polymer such as polypyrrole and Brown liquor, a waste product from paper processing, which contains lignin derivatives. This combination showed excellent charge storage ability due to the synergic combination of lignin and polypyrrole.46
Last but not least, several aspects of redox polymers in batteries are worth noting, as was recently analyzed in a recent review by Chen et al.47 Polymer electrode materials offer electrochemical properties that are not easily accessible for conventional inorganic intercalation compounds such as high theoretical gravimetric capacity (reversible capacities of 300–800 mA h g−1), high rate capacity, wide range of available materials with different operating potentials, processability, low toxicity and potential recyclability. However, it should be noted that it is still challenging for polymer electrodes to achieve high energy/power density and cycling stability simultaneously. In any case, after three decades of research there are still needs for new developments and a deeper understanding of the charge/ion transport mechanism and new approaches to improve electrode stability. Furthermore, there are plenty of possibilities for using the polymers in different battery configurations and types of chemistry. So far, the most common approach involved the use of the polymer as a cathode in lithium metal or lithium ion batteries. However, it is possible to design batteries involving polymers as cathodes as well as anodes using different counter electrodes and electrolyte chemistry (Li, Na, S, Zn, Mg). Formulating these batteries, electrodes and electrolytes adds plenty of complexity to the field and make the comparison among polymers difficult sometimes. It is worth concluding by saying that it is commonly accepted that organic/polymeric materials will penetrate the field of electrode materials for batteries in the near future, since they have already made considerable progress in other electronic and optoelectronic technologies.
Other energy devices: biofuel cells and solar cells
A biofuel cell is an electrochemical device that converts chemical into electrical energy using enzymes as catalysts and simple, natural substances, e.g. glucose as fuel (Fig. 7). Enzyme-based biofuel cells provide a versatile means to generate electrical power from biomass or biofuel substrates, and to use biological fluids as fuel-sources for the electrical activation of implantable electronic medical devices, or prosthetic aids. Efficient energy conversion in a biofuel cell requires the effective immobilisation of enzymes in redox-active hydrogels associated with electrodes.48 Redox polymers are currently implemented for the electrical contacting of oxidases and dehydrogenases with electrodes acting as anodes of biofuel cells, and for the electrical wiring of bilirubin oxidase, cytochrome oxidase, and laccase with electrodes, that yield the cathode units of the biofuel cells.49 Redox polymers act as mediators in order to shuttle electrons between the protein and the electrode surface. Due to a direct electron transfer, the incorporation of redox sites within these proteins is generally not possible given the distance of the active site from the electrode surface. Osmium-based redox polymers have shown to be the most promising candidates to act as mediators in cathodes of membrane-less biofuel cells.50 Different strategies are being developed in this area such as the synthesis of new osmium based redox polymers to cover a broad potential range, or the immobilization of osmium redox polymers on surfaces together with the enzyme using layer-by-layer processes.51 | ||
| Fig. 7 Scheme of an enzyme-based biofuel cell. | ||
On the other hand, dye sensitized solar cells (DSSC) with more than 10% conversion efficiency are nowadays readily achieved with novel ideas and through intensive research.52 Many advantages such as low production cost and diversity in manufactures have made DSSC promising as next-generation photovoltaic devices. One key component of the high performance solar cells is a triiodide/iodide redox couple usually dissolved in an organic liquid electrolyte. Nowadays, DSSC technology faces high-temperature stability issue (>85 °C) to pass standardized packaging durability tests of solar cells. One of the solutions is the substitution of the iodide redox couple or its solidification.53 For this reason, a number of polymers with redox properties have been developed during the last few years aiming at the development of high performance solid dye sensitized solar cells.54 One of the most investigated strategies includes the development of polymers where the iodide redox couple is incorporated such as poly(vinyl imidazolium) iodide polymeric ionic liquids. In another interesting strategy, PEDOT has also been successfully proposed due to its redox properties as a solid substitute of the iodine liquid electrolytes (Fig. 8).55 The best performances of these solid solar cells are still low (approx. 7%) but show some processing advantages, no-need for platinum catalysts and good cyclability. The search for other redox polymers for solid DSSC is a topic of current interest.
 | ||
| Fig. 8 Scheme of a dye sensitized solar cell. | ||
Sensors and biosensors
Sensors and biosensors have been historically one of the most popular application areas of redox polymers. Thus over the years these materials have played a crucial role in the development of electrochemical biosensors as well as chemical and some optical sensors. Glucose electrochemical biosensors are probably the most popular examples as the one represented in Fig. 9. The redox polymer is key in the effective immobilisation of enzymes onto electrodes as a mediator facilitating the electronic flux between the protein and the electrode surface and increasing the sensitivity of the sensor. A number of redox polymers including conducting polymers such as polypyrrole, osmium containing redox polymers or ferrocene modified redox polymers are being investigated.35,56 Cyclic voltammetry studies of the deposited redox polymers usually show an increase in the current with the concentration of glucose in the media, making them suitable as glucose biosensors.57 Trends include the preparation of polymers which lead to the design of biosensors with improved sensitivity and selectivity towards different analytes.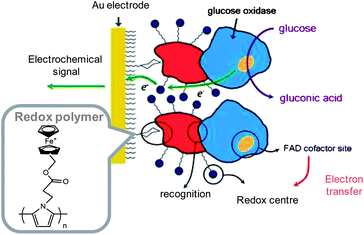 | ||
| Fig. 9 Scheme of a glucose electrochemical biosensor. | ||
The previous paragraph about electrochemical glucose biosensors can be extended to many different electrochemical biosensors which are actually under development. Electrochemical biosensors to detect dopamine, ascorbic acid, hydrogen peroxide, glutamic acid, and DNA hybridization make use of polymers with redox activity in a similar way and in some cases without the need for a specific enzyme.
On the other hand, several metallopolymers have been prepared for sensing applications such as oxygen, pH, metal cations and biomolecules. Since metallopolymers have intrinsic redox properties in most cases it is worth mentioning here their sensing ability although the sensing mechanism is not fully associated with oxidation/reduction processes.58
Electrochromic devices
Electrochromism is the phenomenon exhibited by some materials, which reversibly change their colour when a potential is applied. Redox polymers are in most cases electrochromic materials since they present a change in their optical properties between their reduced and oxidized states due to the chemical changes associated with the gain or loss of electrons. Electrochromic materials are used in devices where their optical properties (color, transparency) are switchable by applying an electrical potential. They are applied in optical displays, smart windows for buildings or automotive applications. A classic electrochromic device configuration is shown in Fig. 10. | ||
| Fig. 10 Schematic representation of an electrochromic device. | ||
Research in electrochromic materials has been historically dominated by inorganic materials such as NiO or WO3. Among the organic materials, conducting polymers have been investigated over the years but initial devices presented cyclability and durability issues. In the last decade with the emergence of stable conducting polymers such as PEDOT, and the development of new conjugated polymers and stable ionic liquid electrolytes these devices have significantly improved extending their cyclability and the range of available color changes.59,60 Trends in electrochromic polymers include the search for polymers which show electrochromic behavior not only in the UV–vis spectra but also in the near-IR region by combining stable polythiophene backbones with other electrochromic redox moieties such as viologens, naphthalene diimide and phenazine.61–63 These polymers show multiple color changes in the same material (i.e. viologen unit color changes and semiconducting polymer color change) and complex electrochemical behaviors as the one illustrated in Fig. 4c.
Nanomedicine: drug delivery and actuators
In the last five years, redox-responsive materials have been attracting much interest in the biomedical field due to their emerging applications in drug and gene delivery systems and actuators such as artificial muscles and valves.64,65 More in particular some pioneering works have appeared in the scientific literature related to the development of redox-responsive nanoparticles or nanocapsules.21,66 These nanoparticles were used as a carrier to encapsulate hydrophobic drugs whose release could be triggered and specifically controlled by a redox reagent (Fig. 11). As a general strategy, disulfide bonds or ferrocene moieties were included in classical polymer microparticles or in block copolymer nanoparticles in order to impart the redox properties. The fact that the drug release can be activated using a redox process or reactant presents a new drug delivery strategy which is expected to be further developed in the next years into a larger variety of systems.67,68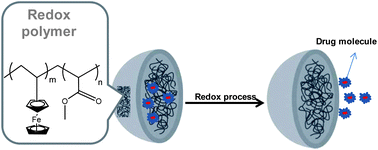 | ||
| Fig. 11 Scheme of a nanocapsule for drug delivery. | ||
Another important application area of redox polymers is actuators. Historically, conducting polymers such as polypyrrole or PEDOT have been used to design electrochemical actuators capable of converting electrochemical stimuli into a mechanical response. This was due to the ionic migration associated with the doping/dedoping mechanism of the redox active conducting polymer. The change of the volume of the polymer structure could be transferred into a mechanical response.69,70
On the other hand, in the last few years two new concepts for actuators based on redox polymers have been introduced. In one example, Vancso et al. published the synthesis of polyferrocenylsilane hydrogels whose polymer–solvent interactions (swelling or deswelling of the gel) can be electrochemically tuned.71 This electrochemical control of the polymer–water interactions is translated in the swelling or deswelling of the hydrogel as the basis for the redox-control mechanical actuation (Fig. 12).
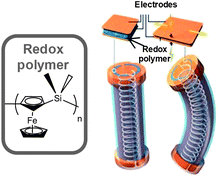 | ||
| Fig. 12 Scheme of a redox-controlled mechanical actuator. | ||
A second new actuation concept was recently presented by Meyer et al. This concept is based on the electroplastic elastomer hydrogels (EPEHs) prepared including the well-known Fe2+/Fe3+ redox couple and polymers functionalized with sulphonate and carboxylate anions.72 The preference of the Fe3+ oxidation state (vs. Fe2+) to bind hard ligands (such as carboxylates) allows the electrochemical control of the cross-linking density resulting in mechanical and physical changes of the hydrogel. As a consequence the new macroscale electroplastic elastomer can be reversibly cycled through soft and hard states while maintaining a three dimensional shape by sequential application of oxidative and reductive potentials.
Materials science
We did not want to finish this mini-review without including the use of redox polymers as reactants for the synthesis of new materials. One clear example is the area of block copolymers including a polyferrocenylsilane backbone due to its wide applications as precursors of catalytically active or magnetic iron nanoparticles.16 Another example is the use of polymeric ionic liquids with oxidant persulphate counter-anions which could be used simultaneously to synthesize and stabilize PEDOT aqueous and organic colloidal dispersions. It is worth noting that the use of polymers as redox active reagents which can also act as macromolecular surfactants is an unexplored interesting option for the design of new types of organic and inorganic colloids.73Redox moieties can also be chemically introduced into polymers in order to design new types of functional materials. In one recent example, redox active biodegradable elastomeric materials have been introduced by Nottelet et al.74 In this work, poly(ε-caprolactone) was crosslinked by thiol–ene chemistry with disulfide bonds in the cross-links. The redox active cross-linker induces elastomeric properties in the materials. In another example, polyacrylamide solubility was controlled in a smart and reversible way by introducing TEMPO moieties. The change in the solubility properties of TEMPO by oxidation/reduction influences changes in the LCST behavior of polyacrylamide from 18 to 35–40 °C which makes them interesting for in vivo drug delivery.75
Concluding remarks
This mini-review highlights the trends and recent advances in the chemistry of redox polymer research. The development of new redox polymers is clearly dominated by their interest in the burgeoning areas of batteries and biosensor research activities. Furthermore, new applications mainly in the energy and biomedical fields indicate a bright present and future for polymers with redox properties. The increasing use of compounds coming from biomass and advances in their macromolecular engineering are expected to have important impact on the development of new polymers with redox properties.Acknowledgements
Financial support of the Basque Government and the European Research Council by Starting grant iPes 306250 is acknowledged.Notes and references
- K. Nakahara, K. Oyaizu and H. Nishide, Chem. Lett., 2011, 222 Search PubMed.
- M. Aydın, B. Esat, Ç. Kiliç, M. Köse, A. Ata and F. Yılmaz, Eur. Polym. J., 2011, 47, 2283 Search PubMed.
- G. Hauffman, J. Rolland, J.-P. Bourgeois, A. Vlad and J.-F. Gohy, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 101 Search PubMed.
- K. Oyaizu, A. Hatemata, W. Choi and H. Nishide, J. Mater. Chem., 2010, 20, 5404 RSC.
- Z. Song, H. Zhan and Y. Zhou, Chem. Commun., 2009, 448 RSC.
- Z. Song, H. Zhan and Y. Zhou, Angew. Chem., Int. Ed., 2010, 49, 8444 CrossRef CAS.
- C. Colombi, D. Comoretto, C. Cuniberti, G. Musso, P. Piaggio and G. Dellepiane, Macromol. Chem. Phys., 1996, 197, 1241 CrossRef CAS.
- S. Luzzati, I. Moggio, D. Comoretto, C. Cuniberti and G. Dellepiane, Synth. Met., 1998, 95, 47 CrossRef CAS.
- M. Yao, H. Senoh, T. Sakai and T. Kiyobayashi, J. Power Sources, 2012, 202, 364 CrossRef CAS.
- E.-N. Esmer, S. Tarkuc, Y. A. Udum and L. Toppare, Mater. Chem. Phys., 2011, 131, 519 Search PubMed.
- J. Y. Zhang, L. B. Kong, L. Z. Zhan, J. Tang, H. Zhan, Y. H. Zhou and C. M. Zhan, J. Power Sources, 2007, 168, 278 CrossRef CAS.
- T. Sarukawa and N. Oyama, J. Electrochem. Soc., 2010, 157, F23 CrossRef CAS.
- S. Inagi, K. Naka and Y. Chujo, J. Mater. Chem., 2007, 17, 4122 RSC.
- J. Bigot, B. Charleux, G. Cooke, F. Delattre, D. Fournier, J. Lyskawa, F. Stoffelbach and P. Woisel, Macromolecules, 2010, 43, 82 CrossRef CAS.
- C. Tonhauser, A. Alkan, M. Schömer, C. Dingels, S. Ritz, V. Mailänder, H. Frey and F. R. Wurm, Macromolecules, DOI:10.1021/ma302241w.
- M. Mazurowski, M. Gallei, J. Li, H. Didzoleit, B. Stühn and M. Rehahn, Macromolecules, 2012, 45, 8970 Search PubMed.
- F. H. Schacher, P. A. Rupar and I. Manners, Angew. Chem., Int. Ed., 2012, 51, 7898 Search PubMed.
- M. A. Hempenius, C. Cirmi, F. L. Savio, J. Song and G. J. Vancso, Macromol. Rapid Commun., 2010, 31, 772 CrossRef CAS.
- X. Sui, M. A. Hempenius and G. J. Vancso, J. Am. Chem. Soc., 2012, 134, 4023 Search PubMed.
- C. Tonhauser, M. Mazurowski, M. Rehahn, M. Gallei and H. Frey, Macromolecules, 2012, 45, 3409 Search PubMed.
- R. H. Staff, M. Gallei, M. Mazurowski, M. Rehahn, R. Berger, K. Landfester and D. Crespy, ACS Nano, 2012, 6, 9042 Search PubMed.
- L. Ren, J. Zhang, C. G. Hardy, S. Ma and C. Tang, Macromol. Rapid Commun., 2012, 33, 510 CrossRef CAS.
- A. A. Jahnke, B. Djukic, T. M. McCormick, E. B. Domingo, C. Hellman, Y. Lee and D. S. Seferos, J. Am. Chem. Soc., 2013., 135, 951 Search PubMed.
- M. Al-Hashimi, M.-A. Baklar, F. Colleaux, S. E. Watkins, T. D. Anthopoulos, N. Stingelin and M. Heeney, Macromolecules, 2011, 44, 5194 CrossRef CAS.
- B. Kim, J. Kim and E. Kim, Macromolecules, 2011, 44, 8791 CrossRef CAS.
- Y. Morisaki, Y. Aiki and Y. Chujo, Macromolecules, 2003, 36, 2594 CrossRef CAS.
- J. Crassous and R. Réau, Dalton Trans., 2008, 6865 RSC.
- E. Rivard, Annu. Rep. Prog. Chem., Sect. A: Inorg. Chem., 2012, 108, 315 RSC.
- P. Pickup, J. Mater. Chem., 1999, 9, 1641 RSC.
- C.-L. Ho and W.-Y. Wong, Coord. Chem. Rev., 2011, 255, 2469 CrossRef CAS.
- X. de Hatten, D. Asil, R. H. Friend and J. R. Nitschke, J. Am. Chem. Soc., 2012, 134, 19170 Search PubMed.
- H. Kang, R. Liu, H. Sun, J. Zhen, Q. Li and Y. Huang, J. Phys. Chem. B, 2012, 116, 55 Search PubMed.
- P. Novák, K. Müller, K. Santhanam and O. Haas, Chem. Rev., 1997, 97, 207 CrossRef CAS.
- C. G. Cameron and P. G. Pickup, Chem. Commun., 1997, 303 RSC.
- D. A. Foucher, C. H. Honeyman, J. M. Nelson, B. Z. Tang and I. Manners, Angew. Chem., Int. Ed., 1993, 32, 1709 CrossRef.
- J. Li, K.-H. Ong, P. Sonar, S.-L. Lim, G.-M. Ng, H.-K. Wong, H.-S. Tan and Z.-K. Chen, Polym. Chem., 2013, 4, 804 RSC.
- U. Lange and V. M. Mirsky, Anal. Chim. Acta, 2012, 744, 29 Search PubMed.
- S. J. Visco, M. Liu and L. C. D. Jongue, J. Electrochem. Soc., 1990, 137, 1191 Search PubMed.
- L. Zhan, Z. Song, J. Zhang, j. Tang, H. Zhan, Y. Zhou and C. Zhan, Electrochim. Acta, 2008, 53, 8319 CrossRef CAS.
- Y. Xuan, M. Sandberg, M. Berggren and X. Crispin, Org. Electron., 2012, 13, 632 Search PubMed.
- H. Nishide, K. Koshika and K. Oyaizu, Pure Appl. Chem., 2009, 81, 1961 CrossRef CAS.
- T. Janoschka, M. D. Hager and U. S. Schubert, Adv. Mater., 2012, 24, 6397 Search PubMed.
- W. Walker, S. Grugeon, O. Mentre, S. Laruelle, J.-M. Tarascon and F. Wudl, J. Am. Chem. Soc., 2010, 132, 6517 CrossRef CAS.
- T. Nokami, T. Matsuo, Y. Inatomi, N. Hojo, T. Tsukagoshi, H. Yoshizawa, A. Shimuzu, H. Kuramoto, K. Komae, H. Tsuyama and J.-I. Yoshida, J. Am. Chem. Soc., 2012, 134, 19694 Search PubMed.
- Z. Song, T. Xu, M. L. Gordin, Y.-B. Jiang, I.-T. Bae, Q. Xiao, H. Zhan, J. Liu and D. Wang, Nano Lett., 2012, 12, 2205 CrossRef CAS.
- G. Milczarek and O. Inganäs, Science, 2012, 335, 1468 CrossRef CAS.
- Y. Liang, Z. Tao and J. Chen, Adv. Energy Mater., 2012, 2, 742 Search PubMed.
- J. W. Gallaway and S. A. C. Barton, J. Am. Chem. Soc., 2010, 130, 8527 Search PubMed.
- F. Barrière, Y. Ferry, D. Rochefort and D. Leech, Electrochem. Commun., 2004, 6, 237 CrossRef.
- P. Scodeller, R. Carballo, R. Szamocki, L. Levin, F. Forchiassin and E. J. Calvo, J. Am. Chem. Soc., 2010, 132, 11132 CrossRef CAS.
- S. Pöller, Y. Beyl, J. Vivekananthan, D. A. Guschin and W. Schuhmann, Bioelectrochemistry, 2012, 87, 178 Search PubMed.
- M. Wang, C. Gräztel, S. M. Zakeeruddin and M. Gräztel, Energy Environ. Sci., 2012, 5, 9394 RSC.
- Y. Kim, Y.-E. Sung, J.-B. Xia, M. Lira-Cantu, N. Masaki and S. Yanagida, J. Photochem. Photobiol., A, 2008, 193, 77 CrossRef CAS.
- J. Xia, N. Masaki, M. Lira-Cantu, Y. Kim, K. Jiang and S. Yanagida, J. Am. Chem. Soc., 2008, 130, 1258 CrossRef CAS.
- E. Azaceta, R. Marcilla, A. Sanchez-Diaz, E. Palomares and D. Mecerreyes, Electrochim. Acta, 2010, 56, 42 Search PubMed.
- M. Şenel, Synth. Met., 2011, 161, 1861 Search PubMed.
- H. Kan, R. Liu, H. Sun, J. Zhen, Q. Li and Y. Huang, J. Phys. Chem. B, 2012, 116, 55 Search PubMed.
- S.-J. Liu, Y. Chen, W.-J. Xu, Q. Zhao and W. Huang, Macromol. Rapid Commun., 2012, 33, 461 CrossRef CAS.
- J. Hwang, I. Schwendeman, B. C. Ihas, R. J. Clark, M. Cornick, M. Nikolou, A. Argun, J. R. Reynolds and D. B. Tanner, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 195121 Search PubMed.
- M. Döbbelin, R. Marcilla, C. Pozo-Gonzalo and D. Mecerreyes, J. Mater. Chem., 2010, 20, 7613 RSC.
- M. Krompiec, I. Grudzka, M. Filapek, Ł. Skorka, S. Krompiec, M. Łapkowski, M. Kania and W. Danikiewicz, Electrochim. Acta, 2011, 56, 8108 Search PubMed.
- L.-P. Gao, G.-J. Ding, C.-L. Li and Y.-C. Wang, Appl. Surf. Sci., 2011, 257, 3039 Search PubMed.
- H. C. Ko, S. Kim, H. Lee and B. Moon, Adv. Funct. Mater., 2005, 15, 905 CrossRef CAS.
- Y. Zhang, S. Kato and T. Anazawa, Smart Mater. Struct., 2007, 16, 2175 Search PubMed.
- Z. Liu and P. Calvert, Adv. Mater., 2000, 12, 288 CrossRef CAS.
- K. Ariga, Y. M. Lvov, K. Kawakami, Q. Ji and J. P. Hill, Adv. Drug Delivery Rev., 2011, 63, 762 CrossRef CAS.
- K. Wang, G.-F. Luo, Y. L. Liu, C. Li, S.-X. Cheng, R.-X. Zhuo and X.-Z. Zhang, Polym. Chem., 2012, 3, 1084 RSC.
- M. Li, Z. Tang, H. Sun, J. Ding, W. Song and X. Chen, Polym. Chem., 2013, 4, 1199 RSC.
- C. Plesse, F. Vidal, D. Teyssié and C. Chevrot, Chem. Commun., 2010, 46, 2910 RSC.
- T. F. Otero and J. G. Martinez, J. Mater. Chem. B, 2013, 1, 26 RSC.
- M. A. Hempenius, C. Cirmi, F. Lo Savio, J. Song and G. J. Vancso, Macromol. Rapid Commun., 2010, 31, 772 CrossRef CAS.
- P. Calvo-Marzal, M. P. Delaney, J. T. Auletta, T. Pang, N. M. Perry, L. M. Weiland, D. H. Waldeck, W. W. Clark and T. Y. Meyer, ACS Macro Lett., 2012, 1, 204 Search PubMed.
- R. Marcilla, E. Ochoteco, C. Pozo-Gonzalo, H. Grande, J. A. Pomposo and D. Mecerreyes, Macromol. Rapid Commun., 2005, 26, 1122 CrossRef CAS.
- M. Nottelet, G. Tambutet, Y. Bakkour and J. Coudane, Polym. Chem., 2012, 3, 2956 RSC.
- H. Fu, D. M. Policarpio, J. D. Batteas and D. E. Bergbreiter, Polym. Chem., 2010, 1, 631 RSC.
| This journal is © The Royal Society of Chemistry 2013 |
