Brønsted acid–base ionic liquids and their use as new materials for anhydrous proton conductors
Md. A. B. H. Susana, Akihiro Nodaa, Shigenori Mitsushimab and Masayoshi Watanabe*a
aDepartment of Chemistry and Biotechnology, Yokohama National University, 79-5 Tokiwadai, Hodogaya-ku, Yokohama 240-8501, Japan. Tel: +81-45-339-3955; Fax: +81-45-339-3955; E-mail: mwatanab@ynu.ac.jp
bDepartment of Energy and Safety Engineering, Yokohama National University, 79-5 Tokiwadai, Hodogaya-ku Yokohama 240-8501, Japan
First published on 18th March 2003
Abstract
Novel Brønsted acid–base ionic liquids, derived from a simple combination of a wide variety of organic amines with bis(trifluoromethanesulfonyl) amide are electroactive for H2 oxidation and O2 reduction at a Pt electrode under non-humidifying conditions, which shows the prospect of the use of protic ionic liquids as new materials for anhydrous proton conductors at elevated temperatures.
The realization that the constraints, associated with the use of water at temperatures higher than 100 °C, place a stumbling block on the widespread use of proton exchange membrane fuel cells,1 has resulted in an upsurge of interest in the option of ubiquitous ionic liquids.2 However, positive results on the utilization of ionic liquids in fuel cell electrolytes have not yet been achieved. Ionic liquids, due to, inter alia, their immeasurably low vapour pressure, high ionic conductivity and greater thermal and electrochemical stability are very promising, but need to act as proton solvents or themselves be capable of conducting protons for their true use in proton conductors. With the aim towards innovating proton conducting ionic liquids for anhydrous proton conductors at elevated temperatures, we, therefore, explored a very expedient way to prepare a novel series of protic ionic liquids and electrochemically provide insight into the proton conduction.
In this study, we used a wide variety of organic amines as Brønsted bases to have neutral salts by a simple combination with a super strong acid, bis(trifluoromethanesulfonyl) amide (HTFSI) under solvent-free conditions. Stoichiometric amounts of HTFSI and amines were mixed and heated above the respective melting points in an argon atmosphere glove box (VAC, [O2]<1 ppm, [H2O]<1ppm).
Most of the amines formed equimolar salts with HTFSI with the exceptions of carbazole, indole, triphenylamine, pyrrole and 1,3,5-triazine. The thermal properties of the neutral salts have been summarized in Table 1. The thermogravimetric (TG) curves of the neutral salts show single-step weight loss processes with sufficient thermal stability (up to >400 °C for 4,4′-trimethylene dipiperidine) as ionic liquids. The formation of neutral salts for some of the low melting point salts was further evinced from the 1H NMR spectra relative to external DSS in D2O solvent using a double tube. It is noteworthy that the neutral salts of 1,2,4-triazole, triethylamine and butylamine are liquid at room temperature. The melting points of the salts varied irrespective of the number of proton acceptor sites and the melting points of the starting amines.3
| Organic Amine (mp/°C)a | Molar Ratio, [Amine]:[HTFSI] | Tm/°Cb | Td/°Cc | σ at 130 °C /10−2 Scm−1d |
|---|---|---|---|---|
| a Data in parentheses are melting points taken from literature.3ab Onset of an endotherm peak (melting point, Tm) during heating scans from −150 °C using differential scanning calorimetry (DSC).c Temperature of 10% weight loss during heating scans from room temperature using TG.d Ionic conductivity, σ was determined by complex impedance method in the frequency range of 5 Hz to 13 MHz at AC amplitude of 10 mV.e Not measured. | ||||
| Pyrrolidine (−58) | 1∶1 | 35.0 | 373 | 3.96 |
| Pyridine (−42) | 1∶1 | 60.3 | 314 | 3.04 |
| Piperidine (−13) | 1∶1 | 37.9 | 363 | 2.35 |
| Acridine (108) | 1∶1 | 116.1 | 353 | —e |
| Butylamine (−50) | 1∶1 | 16.2 | 352 | 1.04 |
| Dibutylamine (−62) | 1∶1 | 42.6 | 325 | 1.26 |
| Triethylamine (−115) | 1∶1 | 3.5 | 350 | 3.23 |
| Diphenylamine (52) | 1∶1 | 51.5 | 193 | 0.85 |
| Imidazole (89) | 1∶1 | 73.0 | 379 | 2.71 |
| Pyrazole (67) | 1∶1 | 58.9 | 265 | 2.65 |
| Pyrazine (54) | 1∶1 | 53.6 | 229 | 3.38 |
| Piperazine (108) | 1∶1 | 172.7 | 358 | —e |
| Benzimidazole (173) | 1∶1 | 101.9 | 368 | 1.31 |
| Morpholine (−7) | 1∶1 | 58.5 | 349 | 1.08 |
| Quinoxaline (29) | 1∶1 | 74.1 | 244 | 1.65 |
| 4,4′-Trimethylenedipyridine (57) | 1∶2 | 62.0 | 386 | 1.05 |
| 4,4′-Trimethylenedipiperidine (66) | 1∶2 | 167.3 | 403 | —e |
| 1,2,4-Triazole (119) | 1∶1 | 22.8 | 287 | 2.20 |
| 1,2,3-Benzotriazole (96) | 1∶1 | 136.6 | 230 | —e |
The equimolar salts exhibited high conductivity (Table 1). The magnitude of ionic conductivity at 130 °C is interestingly higher in the case of the neutral salts of pyrazine, pyridine, pyrrolidine and triethylamine with HTFSI than the corresponding salt of the typical case of imidazole (Im).4 The correlation of various physicochemical properties of the neutral salts with the molecular structure, geometry and basicity of the organic amines is now underway and will be reported elsewhere.
For proton conduction in the neutral salt systems by Grotthuss mechanism,5 the protonated amines require free proton acceptor sites for proton exchange as it is evident for the systems comprised of amines along with their protonated counterparts.4,6 It is therefore of considerable interest to see whether the neutral salts, without stoichiometric excess of free amines can exhibit proton conductivity by functioning of the imide ion (TFSI−) as a proton acceptor site or translational dynamics of the protonated amines (vehicle mechanism7) or hopping through free amines probably persisting in the systems from the equilibrium between neutral salt and the starting amine and HTFSI.
To corroborate protonic conduction in protic ionic liquids, we therefore took Im/HTFSI neutral salt as a representative and conducted a simple direct current polarization experiment at 130 °C using a U-shaped glass tube with two Pt-wire electrodes (proton pump cell). The anode was under H2 or N2 bubbling atmosphere. The current detected under N2 atmosphere was quite low, whereas a noticeable change was eminent upon change to a H2 gas atmosphere resulting in observation of higher current. Furthermore, evolution of gas (H2) was confirmed as bubbles at the cathode. This pinpoints the following phenomenon, occurring at the anode, electrolyte, and cathode:
| Anode: H2
+ 2 Im → 2 HIm+
+ 2 e− Electrolyte: proton conduction Cathode: 2 HIm+ + 2 e− → 2 Im + H2 |
To explore electrode reactions of the neutral salt at the three-phase boundary of the ionic liquid/Pt/H2 or O2, cyclic voltammetric (CV) measurements were conducted using a two-compartment glass cell under dry Ar, H2 or O2 gas bubbling atmosphere (Inset of Fig. 1) by a Solartron electrochemical interface at 130 °C. CV behavior is shown in Fig. 1. In these experiments, Pt and Pt-black electrodes immersed in the neutral salt worked as a working electrode (W.E.) and a counter electrode (C.E.), respectively, and a Pt electrode with H2 bubbling as a reference electrode (R.E.). The cell potential was scanned from the open circuit potential (OCP) to −0.1 V for the first scan followed by scanning to +1.5 V and finally to the OCP. When the W.E. is in Ar atmosphere, the voltammograms show remarkable reduction and oxidation currents at around 0 V. Gas bubbles could be observed on the W.E. in the reduction process at the hydrogen redox potential of 0 V. The R.E., therefore, can be considered as a reversible hydrogen electrode (RHE). Im/HTFSI neutral salt and H2 are electroactive with Pt, and the electrochemical equilibrium reached at the R.E. is
| 2 HIm+ + 2 e− ⇄ 2 Im + H2 |
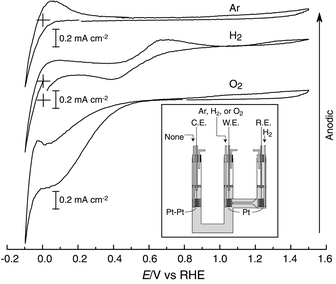 | ||
| Fig. 1 Cyclic voltammograms for Im/HTFSI neutral salt at 130 °C. The crosses correspond to ‘zero’ current at the potential of 0 V. Scan rate is 50 mV s−1. W.E. is a Pt-wire in Ar, H2 or O2 atmosphere, C.E. is a Pt-black wire in H2 atmosphere and R.E. is a Pt-wire in H2 atmosphere. Inset shows the schematic diagram of the two-compartment cell used. | ||
Upon change in the working atmosphere from Ar to H2, large oxidation currents were detected after the re-oxidation. The steady-state H2 oxidation on the Pt working electrode gave rise to the oxidation currents. The H2 bubbles could be visually observed on the counter electrode. We consider these results to be comprehensible and decisive evidence of the proton conduction for the neutral salt. At present, we cannot explain the two-step CV behavior in H2 atmosphere.
When the W.E. is under O2 bubbling atmosphere, a radical change in the shapes of the CVs, as shown in Fig. 1, is observed. The cathodic current is observed at a potential below 0.8 V vs. RHE, and the waves, corresponding to the evolution and reoxidation of H2, are overlapped below 0 V vs. RHE. The cathodic current, judged from the potential, appears to be assigned to the 4-electron O2 reduction reaction. Since the proton carrier in this system is HIm+, the reaction can be expressed as
| O2 + 4 ImH+ + 4e− → 2 H2O + 4 Im |
Fig. 2 represents current vs. potential (vs. RHE) characteristics of H2/O2 fuel cells under non-humidifying conditions. The measurement was conducted at 130 °C using the glass cell in Fig. 1, with the working and counter electrodes under O2 and H2 bubbling atmosphere. Although a potential drop with increasing current density is apparent possibly due to the cathodic polarization and to some extent to IR drop, this is, to our knowledge, the first evidence of electric power generation by an H2/O2 fuel cell using a Brønsted acid–base ionic liquid as a proton conducting non-aqueous electrolyte in sharp contrast to the negative result reported on a related material.1b For the sake of clarification, two fuel cells containing Im/HTFSI neutral salt as an electrolyte were connected in series. When H2 and O2 gas were bubbled at the electrodes, power generated by the system at 130 °C could be used for operation of a calculator.
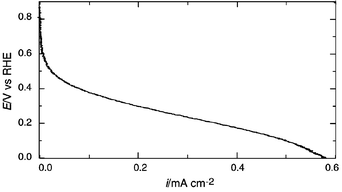 | ||
| Fig. 2 Fuel cell characteristics for Im/HTFSI neutral salt at 130 °C. Scan rate is 1 mV s−1. W.E. is a Pt-wire in O2 atmosphere, C.E. is a Pt-black wire in H2 atmosphere and R.E. is a Pt-wire in H2 atmosphere. | ||
In resemblance to the Im/HTFSI system, some other ionic liquids of this variety also exhibit proton conductivity. The concept of protic ionic liquid may, therefore, be used with compatible polymers for the construction of solid-state proton conductors,8 and further, an acidic site and/or a basic site can be affixed to a polymer backbone. This finding may open up a new field of fuel cells under non-humidifying conditions, which may be operated at temperatures above 100 °C and provide a firm underpinning for further development. In addition, development of fundamental, molecular-based descriptions and models, will aid in tuning the protic ionic liquids to desirable chemistry for potential application in multidisciplinary areas.
This research was supported in part by Grant-in-Aid for Scientific Research (#404/11167234) from the Japanese Ministry of Education, Science, Sports, and Culture and by NEDO Technology Research Grant. M.A.B.H.S. acknowledges a post-doctoral fellowship from JSPS.
Notes and references
- (a) K. D. Kreuer, CHEMPHYSCHEM, 2002, 3, 771 CrossRef CAS; (b) C. Yang, P. Costamagna, S. Srinivason, J. Benziger and A. B. Bocarsly, J. Power Source, 2001, 103, 1 Search PubMed.
- (a) M. Doyle, S. K. Choi and G. Proulx, J. Electrochem. Soc., 2000, 147, 34 CrossRef CAS; (b) J. Fuller and R. T. Carlin, Electrochem. Soc. Proc., 1999, 41, 27 Search PubMed.
- (a) M. Windholz, The Merck Index; An Encyclopedia of Chemicals and Drugs, Merck & Co., Inc: NJ, 9th Ed., 1976 Search PubMed; (b) M. Hirao, H. Sugimoto and H. Ohno, J. Electrochem. Soc., 2000, 147, 4168 CrossRef CAS.
- A. Noda, M. A. B. H. Susan, K. Kudo, S. Mitsushima, K. Hayamizu and M. Watanabe, J. Phys. Chem. B., 2003, in press.
- C. J. D. van Grotthuss, Ann. Chim., 1806, 58, 54 Search PubMed.
- (a) K. D. Kreuer, A. Fuchs, M. Ise, M. Spaeth and J. Maier, Electrochim. Acta, 1998, 43, 1281 CrossRef CAS; (b) M. Schuster, W. H. Meyer, G. Wegner, H. G. Herz, M. Ise, M. Schuster, K. D. Kreuer and J. Maier, Solid State Ionics, 2001, 145, 85 CrossRef CAS; (c) R. Bouchet and E. Siebet, Solid State Ionics, 1999, 118, 287 CrossRef CAS.
- K. D. Kreuer, A. Rabenau and W. Wepner, Angew. Chem., Intl. Ed. Engl., 1982, 21, 208 CrossRef.
- A. Noda and M. Watanabe, Electrochim. Acta, 2000, 45, 1265 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |
