Iron-mediated inter- and intramolecular reductive cross-coupling of unactivated alkyl chlorides with aryl bromides†
Abstract
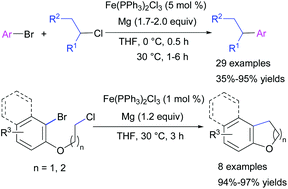
An efficient one-pot intermolecular reductive cross-coupling of unactivated primary and secondary alkyl chlorides bearing β-hydrogens with aryl bromides is described. A combination of magnesium turnings and a catalytic amount of the commercially available iron(III) complex Fe(PPh3)2Cl3 was used, and the conditions were also successfully extended to an intramolecular reaction for the first time. Both types of cross-coupling reactions proceed under mild conditions, involving the in situ generation of aryl Grignard reagents, and show good applicability to a variety of readily available unactivated alkyl chlorides, which have previously been challenging substrates in iron-catalyzed reductive cross-coupling reactions.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery

 Please wait while we load your content...
Please wait while we load your content...