Synthesis and thermodynamics of Ag–Cu nanoparticles
Abstract
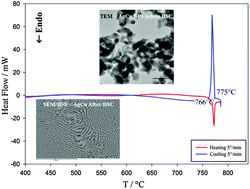
Metallic silver, copper, and Ag–Cu nanoparticles (NPs) have been produced by a chemical reduction method. The obtained nanoparticles were characterized by powder X-ray diffraction (XRD) and transmission electron microscopy (TEM). A side-segregated configuration was observed for the one-pot synthesized Ag–Cu NPs, and the melting temperature depression of about 14 °C was found by differential scanning calorimetry (DSC). A comparison between the new experimental data, the literature data on Ag–Cu bimetallic NPs and the corresponding theoretical values obtained from the Ag–Cu nano-sized phase diagram was done, whereas the melting behaviour of Ag and Cu metal nanoparticles was discussed in the framework of the liquid layer model (LLM).
- This article is part of the themed collection: Recent advances in the chemical physics of nanoalloys

 Please wait while we load your content...
Please wait while we load your content...