Enantioselective synthesis of cyanohydrins catalysed by hydroxynitrile lyases – a review
Abstract
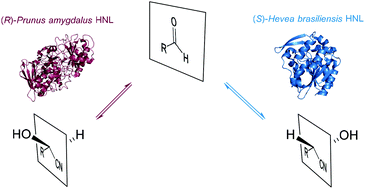
The first enantioselective synthesis was the selective addition of cyanide to benzaldehyde catalysed by a hydroxynitrile lyase (HNL). Since then these enzymes have been developed into a reliable tool in organic synthesis. HNLs to prepare either the (R)- or the (S)-enantiomer of the desired cyanohydrin are available and a wide variety of reaction conditions can be applied. As a result of this, numerous applications of these enzymes in organic synthesis have been described. Here the examples of the last decade are summarised, the enzyme catalysed step is discussed and the follow-up chemistry is shown. This proves HNLs to be part of main stream organic synthesis. Additionally the newest approaches via immobilisation and reaction engineering are introduced.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery

 Please wait while we load your content...
Please wait while we load your content...