Synthesis and absolute configuration assignment of albucidin: a late-stage reductive deiodination by visible light photocatalysis†
Abstract
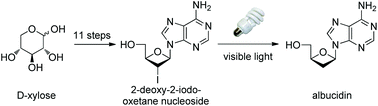
The synthesis of albucidin and its enantiomer are described. It involves a visible-light photocatalysis deiodination at the late stage. The absolute configuration of natural albucidin is determined as (1R,3S). This work provides a basis for structural modification to develop a new type of herbicidal from an old structure.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery

 Please wait while we load your content...
Please wait while we load your content...