Alternative solid-state forms of a potent antimalarial aminopyridine: X-ray crystallographic, thermal and solubility aspects†
Abstract
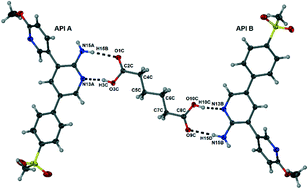
3-(6-Methoxypyridin-3-yl)-5-(4-methylsulfonyl phenyl)-pyridin-2-amine (MMP) is a member of a novel class of orally active antimalarial drugs. This aminopyridine molecule has shown potent in vitro antiplasmodial activity and in vivo antimalarial activity in Plasmodium berghei-infected mice. The aqueous solubility of this molecule is, however, limited. Thus investigations aimed at improving the physicochemical properties, including solubility, of MMP were accordingly conducted. Five salts of MMP were formed with co-former molecules saccharin, salicylic acid, fumaric acid, oxalic acid and suberic acid, but a cocrystal was obtained when the co-former adipic acid was employed. All these new multi-component systems have been fully characterised using X-ray diffraction and thermal methods. Semi-quantitative, turbidimetric solubility tests in a phosphate-buffered saline solution at a pH of 7.4 were performed on the salts and the cocrystal of MMP. The saccharinate salt, fumarate salt and the cocrystal of MMP proved to have greater solubility than MMP itself. This work illustrates the importance of screening and modifying candidate drug compounds in their preliminary stages of development.
- This article is part of the themed collection: Functional Co-crystals

 Please wait while we load your content...
Please wait while we load your content...