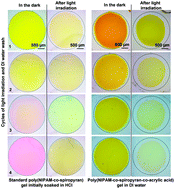
Up to now, photoresponsive hydrogels and ionogels based on poly(N-isopropylacrylamide) copolymerised with pendant spiropyran groups require exposure to external acidic solution (usually milimolar HCl) to generate the swollen gel prior to photo-triggered contraction. This serious functional limitation has been solved by copolymerising acrylic acid into the gel matrix, to provide an internal source of protons. Due to the relative pKa values of acrylic acid and the spiropyran and merocyanine isomers, the protonation and deprotonation occurs internally within the gel and there is no need for an external source of protons. Furthermore, the shrinking–expansion cycles of these gels in deionised water are repeatable, as protonation throughout the gel does not rely on movement of protons from an external acidic solution into the bulk gel. In contrast to previous formulations, these gels do not show degradation of their photo-induced shrinking ability after multiple washings in deionised water and repeated switching over a 2 month period.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...