Reference electrode assembly and its use in the study of fluorohydrogenate ionic liquid silicon electrochemistry
Abstract
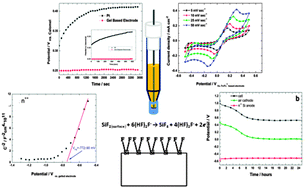
Silicon electrochemistry in fluorohydrogenate ionic liquids is partly hampered owing to the incapability of producing an accurate and reproducible potential measurement due to a lack of appropriate reference electrodes. This research work describes a simple assembly of a stable external reference electrode enabling accurate studies of silicon electrochemistry in fluorohydrogenate ionic liquids. The electrode configuration is based on the ferrocene/ferrocenium (Fc|Fc+) couple dissolved in the EMIm(HF)2.3F (1-ethyl-3-methyl-imidazolium fluorohydrogenate)/Carbopol 941 gel. A stable potential of 2.5 wt% Carbopol-based electrode was measured versus a calomel reference electrode at 250 ± 3 mV. By utilizing the constructed electrode, an intensive electrochemical investigation on n-type silicon in EMIm(HF)2.3F was conducted. Flat-band and open circuit potentials were measured, along with Si–air half- and full-cell electrochemical measurements. A suggested mechanism for the n-type Si dissolution process in the EMIm(HF)2.3F electrolyte, without illumination, is discussed as well.

 Please wait while we load your content...
Please wait while we load your content...