Enantioselective monofluoromethylation of aldehydes with 2-fluoro-1,3-benzodithiole-1,1,3,3-tetraoxide catalyzed by a bifunctional cinchona alkaloid-derived thiourea–titanium complex†
Abstract
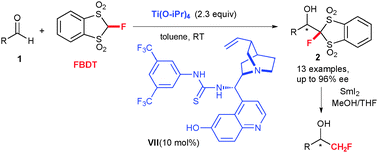
A catalytic enantioselective monofluoromethylation of aldehydes using 2-fluoro-1,3-benzodithiole-1,1,3,3-tetraoxide (FBDT) was realized. With a bifunctional thiourea–titanium catalytic system, the monofluoromethylated-adducts were obtained in good yields and high enantioselectivities, up to 96% ee.

 Please wait while we load your content...
Please wait while we load your content...