Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Concluding remarks: challenges and prospects in organic photonics and electronics†
Hiroyuki
Nishide

Research Institute for Science and Engineering, Waseda University, Tokyo 169-8555, Japan. E-mail: nishide@waseda.jp
First published on 15th December 2023
Abstract
The Faraday Discussion meeting on ‘challenges and prospects in organic and photonics and electronics’ was held in Osaka, Japan, after the COVID pandemic and during the subsequent global difficulties, in the traditional face-to-face and condensed style, with many discussions, both after the short presentations and in front of the poster presentations. I would like to take this opportunity to thank the organising members, particularly Youhei Takeda and local professors, for their efforts in organising this meeting.
I feel privileged to address the concluding remarks of the meeting; however, it is extremely difficult to describe the discussions that emerged from this meeting, both in terms of the wide-ranging research fields considered and the depth of the discussions. Items of the discussions were extended, along the same organic-based avenue of the previous meeting on this topic in 2014,1 to include the frontiers of socially demanded, energy-related applications using organic molecules and polymers, i.e. thermoelectric and battery electrode-active materials. The label ‘organic’ often included metal complexes and molecular composites with carbons, and the results were obtained by effectively using other components in the devices. The specific discussions for the 3 days were recorded; hence, I will share my perspective on the issues raised in this meeting and suggestions, just as one of the references.
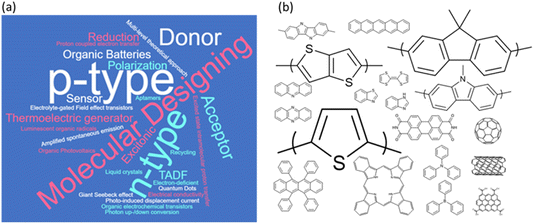
I went through, in advance, the papers contributed to this meeting, and generated a keyword cloud (Fig. 1a) to reflect on our active discussions. The most frequent keywords, p- and n-type, indicate that the discussion was centered on the traditional properties of p- and n-type, or donor and acceptor, semiconducting organics throughout all sessions. A molecular structure cloud is shown in Fig. 1b, wherein polythiophene, polyfluorene, carbazole and bithiophene derivatives were studied in many papers. These are conventional molecules and there is no surprise there. I am concerned about the framework of Faraday Discussions for circulating the speakers’ accepted manuscripts in advance. Short oral presentations were provided with emphasis on the speakers’ concepts and ideas, often including novel keywords and chemical structures; however, I noticed that the papers that will be published in the special issue of this meeting may remain monotonous, despite the active discussions followingly recorded.
Organic-based devices, including photonic and electronic ones, are classified in Table 1, adding energy-related device systems, and contrasting the general dry devices with the wet or electrochemical devices, where there is coexisting electrolyte, in the right column. Electrochemical transistors that include electrolyte-gated organic field-effect transistors were overviewed in this meeting by Luisa Torsi. Her Spiers Memorial Lecture (https://doi.org/10.1039/D3FD00152K) was very impressive, in which she discussed powerful applications of transistor systems to single-molecule biosensing for medical purposes.2 Electrochemical light-emitting cells are considered a substitute for the counterpart organic light-emitting devices (OLEDs) owing to their simple, active layer structure, composed of a fluorescent molecule with an electrolyte, and its cost effectiveness.3 Electrochromic displays, typical wet device systems, were not included in this meeting; they have practical challenges and are still being developed for displays and smart windows on the market by exploring new chromophore molecules and polymers with reversible and persistent redox capability in their electrochemical reactions.4 The organic battery is a newcomer in this meeting,5 and its dry counterpart is an ultrahigh energy-storage capacitor based on novel dielectric polymers.6 An organic thermoelectric generator is paired with a wet device, which forms a thermo-electrochemical cell.
| Dry devices | Electrochemical devices (coexistence of electrolytes) |
|---|---|
| Organic field-effect transistors | Electrochemical transistors |
| Organic light-emitting diodes/displays | Light-emitting electrochemical cells |
| (Liquid crystal displays) | Electrochromic displays |
| Organic photovoltaic cells (incld., perovskite solar cells) | Dye-sensitized solar cells |
| Dielectric polymer capacitors | Organic batteries |
| Organic thermoelectric generators | Thermo-electrochemical cells |
| ⋮ | ⋮ |
Session 1 on organic bioelectronics started with Nako Nakatsuka’s presentation on the surface chemistry of a polymer/carbon nanotube coating with integrated DNA aptamers upon an electrolyte-gated transistor for biomolecule sensing (https://doi.org/10.1039/D3FD00123G). As Luisa Torsi touched on in her talk, an electrochemical understanding of both the electronic–ionic mixed transport in the organic semiconducting layers and the interface of the electrolyte with the analyte is critical.
We can expect bioelectronics to provide cutting-edge approaches to shaping our future chemistry.
From another angle, implementing proteins in optoelectronics represents a fresh idea toward a new class of sustainable materials with biological functions that could replace less-environmentally-friendly inorganic components. As one of the references, a study by Costa et al. is quoted here.7 They screened and selected green fluorescent protein mutants, which increased thermal tolerance and provided fast folding for protein production. The fluorescent proteins were genetically-encoded to aggregate with a hydrophilic polymer, so that they became dehydrated and were integrated into a light-guiding host polymer. The fluorescent protein-based diode is expected to achieve a high emitting performance without thermal quenching, even under high power driving conditions. In addition to the biological and medical applications of such chromophore proteins, there is an expectation that bio-optoelectronics may bridge the gap between organic materials and biological systems.
Our results on the electrical conduction of a material that coexists with electrolyte, but is purely organic, can be considered as another example, which is expected to stimulate research on currently unknown electric and ionic functions of soft organic matter, including biological systems. We prepared flexible polymers bearing pendant redox groups, which delivered an electrical conductivity of 10−4 S cm−1.8 A macroscopic, huge current density beyond 1 mA cm−2 was observed, even for sub-mm thick specimens; when one student accidently touched one, he felt an electric shock. The redox-active polymer functions as an electrically conducting material, and its electric conduction and extremely efficient charge transport are attributed to a sequence of rapid and geared chemical reactions between the densely populated redox sites. This concept is totally different from that of metallic conduction or π-electron-based conduction in organic semiconductors.
Organic photoelectronic-responsive compounds comprise earth-abundant elements and have been described as sustainable materials for use in the next generation of displays and several other devices. However, the practical availability of photoelectronic organics depends on the chemical costs and hazards, i.e. starting compounds, building blocks, synthetic routes, reaction conditions and solvents, by-products, and purification. Specific requirements or assessments for ‘sustainability’ or ‘greenness’ have not yet been settled. For example, Merck/Sigma-Aldrich marks their chemicals with a ‘green label’ among the categories9 that include the twelve principles of green chemistry. Some of our familiar molecules are provided with this green label and advertised; however, I am concerned about their commercial perspective on this categorising process. Christine Luscombe discussed her synthesis of glycolated polythiophenes through a fast route and facile procedure in this session (https://doi.org/10.1039/D3FD00146F). Such a delineation is expected to be one of the first steps to provide a multidimensional picture of the real environmental impacts of organic photoelectronic compounds.
I am confident that this session at the Faraday Discussions will, in the not-far future, leverage the integration of organic photo-electronics between biological, environmental, and energy sciences.
Organic batteries could be a counterpart of the highly in-demand Li-ion batteries, and their chemistry was discussed in Session 2. They are rechargeable devices based on the electrochemical redox reaction of organic molecules or polymers in the presence of electrolytes. The doping process of π-conjugated polymers can be regarded as a reversible redox reaction, and they have been examined as battery electrode-active materials.10 However, the doping levels remain low, resulting in a battery with very low energy density. In addition, the output voltage and the response rate are broadened and fluctuated depending on the doping level; this research was suspended, and now the target is electrochemically reversible redox pairs of organic molecules.
Molecules with more positive redox potentials, or often p-type molecules, are utilised as cathode active compounds (as represented by the triphenylamine/aminium radical cation redox couple), whereas those with more negative potentials, or often n-type ones, are used for the anode (e.g., the quinone/hydroquinone redox couple). The electrochemical reaction occurs in the amorphous or gel-like state of the redox molecules or polymers, which are fabricated in paper-like, flexible batteries (Fig. 2a).11 Research on the target redox polymers is currently underway, considering new redox-active sites and/or higher dimensional structures. Rebeca Marcilla provided an interesting example of this approach, significantly improving the capability of the featured anthraquinone and phenazine network polymers with fast-rate electrodes, applicable to an aluminium battery (https://doi.org/10.1039/D3FD00132F).
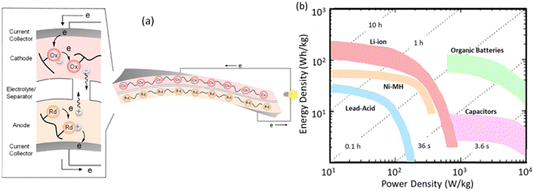 | ||
| Fig. 2 (a) An organic, flexible paper-like battery fabricated using a pair of redox polymers and an electrolyte-containing thin-film separator and (b) batteries and capacitors plotted in a diagram of energy density vs. power density: Ragone plots (the h and s values represent the time required for full charging/discharging). (a) Reproduced from ref. 11b from the Royal Society of Chemistry 2022. | ||
The performance of emerging organic batteries is projected in Fig. 2b, a comparison of the energy density vs. power density of organic batteries with those of the conventional batteries, which represents an advantage in power characteristics; however, there is a drawback in terms of energy density, which is nearly one order of magnitude behind those of the rapidly improving Li-ion batteries. A warning should be noted, that batteries are charging/discharging energy-storage devices ‘electrically’ designed and inspected toward practical usages.
Capacitors function on the physical charge storage at interfaces under external voltage application, and are characterised by quick charging/discharging properties but limited energy density and inconstant output voltage. Kunio Awaga discussed, using a device with a metal–insulator–organic tetrathiafulvalene layer–metal configuration, charge storage at the interface of an organic semiconductor and a photo-induced displacement current to possibly lead to a photoelectronic conversion phenomenon (https://doi.org/10.1039/D3FD00125C). His proposal suggested that organic semiconducting materials show a variety of capacitor-like behaviours. For example, a battery-inspired non-volatile and rewritable memory was reported by sandwiching a dielectric polymer film with a pair of semiconducting polymers.12
In Session 3, the wide fields of excitonic molecules and their applications were reported and discussed. The research on thermally activated delayed fluorescence (TADF)13 progresses both by molecular tailoring of emitters and by analysing dynamic reverse intersystem crossing. For the former, the twisted donor–acceptor configuration was discussed, including the contribution of the structural symmetry and coplanarity. Albrecht et al. synthesised tris(chlorophenyl)methyl radicals substituted with the donor carbazole and presented the unique luminescence property of the organic radical molecules with a doublet electron configuration (https://doi.org/10.1039/D3FD00130J). For the latter, Youichi Tsuchiya discussed the luminescence mechanism of TADF molecules, as well as his recent results on the peculiar thermal behaviour or inverted ΔEST for reverse intersystem crossing (https://doi.org/10.1039/D3FD00151B). Nevertheless, TADF molecules, endowed with robustness, are anticipated for use in next-generation OLEDs, as well as electrically driven organic lasers.14
Switching of the emission channel from TADF to room-temperature phosphorescence is another avenue to enhance the quantum efficiency for OLEDs: e.g., organic emitters have been researched by designing donor–acceptor–donor moieties, as reported by Data et al.15
Jenny Clark described the chemistry of singlet fission with her precise spectroscopy of a series of π-conjugated molecules, including single crystals of rubrene (https://doi.org/10.1039/D3FD00150D). The discussion in this session will stimulate the design of organic molecules for triplet–triplet annihilation and photon-up-conversion. Organic chromophores to enable efficient triplet–triplet annihilation conversion are being explored, as discussed by Yanai et al.,16 along with the molecular design of relatively small but rigid conjugated structures. Their combination with a triplet sensitizer facilitates photon up-conversion from low-intensity visible light into UV light, leading to various photochemical applications.
The questions posed in this session include how we can imagine the next challenge for excitonic molecules in OLEDs and sunlight-powered devices such as organic photovoltaics.
Recent studies on precise model compounds of conjugated organics provide a comprehensive understanding of the extensive characteristics of organic semiconductors. Fujino et al. discussed, in this meeting, carrier–carrier repulsion and interpolymer interactions to dominant p-type conductivity, using single-crystal charge-transfer salts of oligo(ethylenedioxythiophene)s (https://doi.org/10.1039/D3FD00134B). Fukazawa et al.17 recently reported on oligo(biindenylidene)s, one-dimensional fragments of fullerenes, and elucidated their multi-electron reduction capability, as well as enhanced visible absorption; this suggests a new approach to n-type conjugated polymers without any electron-withdrawing substituents. In addition, Skabara et al.18 pointed out, through their crystal structure analysis, the importance of heteroatom interactions in heterocyclic conjugated molecules and polymers.
Organic thermoelectrics were discussed in Session 4, wherein active materials would be configured in thermoelectric generators as p- and n-type conductors connected with high- and low-temperature heat sources. Key materials expected are n-type conjugated polymers, and their development was demonstrated by Xugang Guo through his molecular design strategy (https://doi.org/10.1039/D3FD00135K). He also stressed that his n-type polymers are commonly applicable to photovoltaics and transistors. Bob Schroeder discussed structure–conductivity relationships using his coordination polymers (https://doi.org/10.1039/D3FD00139C). In addition, Nakamura et al. mapped highly pure organic small molecules with a giant Seebeck effect and proposed possible origins of thermoelectric conduction in organic molecules, represented by heat-flow-based molecular vibration (https://doi.org/10.1039/D3FD00127J).
I would like to touch upon the general knowledge that the thermal conductivity of organics, including polymers, remains extremely low, being approximately one order of magnitude lower than those of inorganic ceramics and three orders of magnitude lower than those of metals. Polymers and plastics are thermal insulators. Appropriately lower thermal conductivity is better for thermoelectrics. However, designing and synthesizing highly thermo-conducting organic polymers, genuine and un-composited ones, are significant challenges to solving the crucial problem of heat dissipation or heat removal from plastic packages of operating devices and batteries.
Materials informatics may reduce our tremendous efforts in exploring novel materials. Indeed, machine learning procedures, including deep learning, have been successful not only to predict the properties of organic compounds with important feature values, but also to propose target molecular structures through an inverse-design process. Materials informatics functions more efficiently with feedback from the experimental results based on the prediction. The cycle of the informatics and corresponding experiments could be run via high-throughput syntheses and characterisation (Fig. 3).
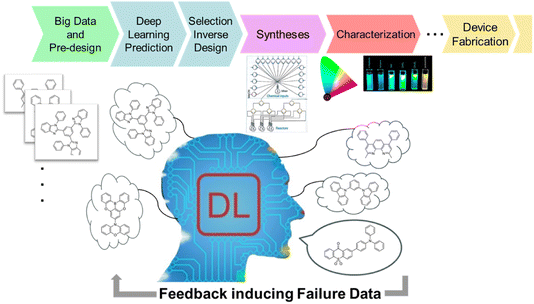 | ||
| Fig. 3 Materials informatics including explainable deep learning (DL) and an ecosystem integrated with high-throughput syntheses and characterization. | ||
However, materials informatics often encounters the problems of a relatively small number of experimental data, e.g. a hundred samples, whereas the trending deep-learning techniques generally require considerably larger numbers of samples, i.e. more than ten thousand. The optical properties of functional chromophore molecules, HOMO and LUMO levels, as well as absorption and emission peak positions and bandwidths, exciton coefficients, photoluminescence quantum yields, and emission lifetime, are important parameters for searching for new organic molecules. In addition, they often involve complicated factors, i.e., amorphous or crystalline and higher-ordered structures, molecular interactions, powders, films, or solutions, and hysteresis in device fabrication and operation.
To challenge the complexity of these organic molecules in materials informatics, several databases have been recently reported, collecting related data on organic molecules from the literature and/or researchers’ experimental results. For example, Park et al. reported a database that included more than 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 organic chromophores and 500 solvents,19 to account, e.g., local environment effects on optical properties. I quote another recent paper published in Nature Energy on perovskite solar cells,20 where a big database was created with almost hundred authors. Unger et al. widely called on the European Union’s Horizon project members to collect data through careful screening with the same guidelines and format from the meaningful 40
000 organic chromophores and 500 solvents,19 to account, e.g., local environment effects on optical properties. I quote another recent paper published in Nature Energy on perovskite solar cells,20 where a big database was created with almost hundred authors. Unger et al. widely called on the European Union’s Horizon project members to collect data through careful screening with the same guidelines and format from the meaningful 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 devices with up to a hundred parameters, including hole- and electron-transporting organic materials, from reliable published papers.
000 devices with up to a hundred parameters, including hole- and electron-transporting organic materials, from reliable published papers.
Several data initiatives in materials science have been under development, e.g. the MatNavi database of the Japanese National Institute of Materials Science. Despite these efforts, several organic chemists are still struggling to overcome the accuracy limitation owing to the low amount of their data. I hope this meeting could be one of the opportunities to talk about how to share your data, to combine and constitute a database of organic electronic and photonic compounds applicable to practical research.
We are living in the age of artificial intelligence. For preparing my final concluding words, I asked Bing Chat, a natural language processing dialogue system: can you write 80 words on ‘challenges and prospects in organic photonics and electronics’?21 The answer was as follows:
“Organic photonics and electronics, while promising for their flexibility, biocompatibility, and tunable properties, face significant challenges. These include issues with stability, efficiency, and scalability that hinder their widespread adoption. However, the field is ripe with potential, with prospective applications ranging from displays and sensors to energy devices. The key to unlocking this potential lies in advancements in material science and device engineering, which could address the existing challenges and open new avenues in the field.”
In conclusion, I am optimistic that engaging in discussions throughout the four sessions will inspire new ideas, and that this discussion platform will remain very vibrant.
Conflicts of interest
There are no conflicts to declare.References
- Themed Collection: Organic Photonics and Electronics, Faraday Discuss., 2014, 174, 1–448 Search PubMed.
- F. Torricelli, D. Z. Adrahtas, Z. Bao, M. Berggren, F. Biscarini, A. Bonfiglio, C. A. Bortolotti, C. D. Frisbie, E. Macchia, G. G. Malliaras, I. McCulloch, M. Moser, T. Nguyen, R. M. Owens, A. Salleo, A. Spanu and L. Torsi, Nat. Rev. Methods Primers, 2021, 1, 66, DOI:10.1038/s43586-021-00065-8; E. Genco, F. Modena, L. Sarcina, K. Björkström, C. Brunetti, M. Caironi, M. Caputo, V. M. Demartis, C. D. Franco, G. Frusconi, L. Haeberle, P. Larizza, M. T. Mancini, R. Österbacka, W. Reeves, G. Scamarcio, C. Scandurra, M. Wheeler, E. Cantatore, I. Esposito, E. Macchia, F. Torricelli, F. A. Viola and L. Torsi, Adv. Mater., 2023, 35, 2304102, DOI:10.1002/adma.202304102.
- L. Mardegan, A. Paliwal, K. P. S. Zanoni, D. Tordera and H. J. Bolink, Adv. Opt. Mater., 2022, 10, 2201953, DOI:10.1002/adom.202201953; K. Sato, S. Uchida, S. Toriyama, S. Nishimura, K. Oyaizu, H. Nishide and Y. Nishikitani, Adv. Mater. Technol., 2017, 2, 1600293, DOI:10.1002/admt.201600293.
- J. R. Reynolds, B. C. Thompson and T. A. Skotheim, Conjugated Polymers, CRC Press, Milton, 2019 RSC; A. M. Österholm, L. Nhon, D. E. Shen, A. M. Dejneka, A. L. Tomlinson and J. R. Reynolds, Mater. Horiz., 2022, 9, 252–260, 10.1039/d1mh01136g.
- K. Oyaizu and H. Nishide, Adv. Mater., 2009, 21, 2339–2344, DOI:10.1002/adma.200803554; B. Esser, F. Dolhem, M. Becuwe, P. Poizot, A. Vlad and D. Brandell, J. Power Sources, 2021, 482, 228814, DOI:10.1016/j.jpowsour.2020.228814; U. S. Schubert, A. Winter and G. R. Newkome, An Introduction to Redox Polymers for Energy-Storage Applications, Wiley-VCH, Weinheim, 2023 Search PubMed.
- T. D. Huan, S. Boggs, G. Teyssedre, C. Laurent, M. Cakmak, S. Kumar and R. Ramprasad, Prog. Mater. Sci., 2016, 83, 236–269, DOI:10.1016/j.pmatsci.2016.05.001; Y. Feng, Y. Hasegawa, T. Suga, H. Nishide, L. Yang, G. Chen and S. Li, Macromolecules, 2019, 52, 8781–8787, DOI:10.1021/acs.macromol.9b01785.
- A. Espasa, M. Lang, C. F. Aguiño, D. Sanchez-deAlcazar, J. P. Fernández-Blázquez, U. Sonnewald, A. L. Cortajarena, P. B. Coto and R. D. Costa, Nat. Commun., 2020, 11, 879, DOI:10.1038/s41467-020-14559-8; M. Patrian, M. Nieddu, J. A. Banda-Vázquez, D. Gutierrez-Armayor, G. González-Gaitano, J. P. Fuenzalida-Werner and R. D. Costa, Adv. Mater., 2023, 2303993, DOI:10.1002/adma.202303993.
- Y. Sasada, R. Ichinoi, K. Oyaizu and H. Nishide, Chem. Mater., 2017, 29, 5942–5947, DOI:10.1021/acs.chemmater.7b01476; K. Hatakeyama-Sato, H. Wakamatsu, R. Katagiri, K. Oyaizu and H. Nishide, Adv. Mater., 2018, 30, 1800900, DOI:10.1002/adma.201800900.
- Merck KGaA, Our Sustainability Report, accessed on 27/Nov/ 2023, https://www.merckgroup.com/en/sustainability.html Search PubMed.
- P. Novák, K. Müller, K. S. V. Santhanam and O. Haas, Chem. Rev., 1997, 97, 207–282, DOI:10.1021/cr941181o; H. Nishide and T. Suga, Electrochem. Soc. Interface, 2005, 14, 32–36, DOI:10.1149/2.F04054IF.
- (a) H. Nishide and K. Oyaizu, Science, 2008, 319, 737–738, DOI:10.1126/science.1151831; (b) H. Nishide, Green Chem., 2022, 24, 4650–4679, 10.1039/d2gc00981a.
- Y. Yonekuta, K. Susuki, K. Oyaizu, K. Honda and H. Nishide, J. Am. Chem. Soc., 2007, 129, 14128–14129, DOI:10.1021/ja075553p.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234, DOI:10.1038/nature11687.
- K. Yoshida, J. Gong, A. L. Kanibolotsky, P. J. Skabara, G. A. Turnbull and I. D. W. Samuel, Nature, 2023, 621, 746–752, DOI:10.1038/s41586-023-06488-5; X. Tang, M. Xie, Z. Lin, K. Mitrofanov, T. Tsagaantsooj, Y. Lee, R. Kabe, A. S. D. Sandanayaka, T. Matsushima, T. Hatakeyama and C. Adachi, Angew. Chem., Int. Ed., 2023, e202315210, DOI:10.1002/anie.202315210.
- H. F. Higginbotham, M. Okazaki, P. de Silva, S. Minakata, Y. Takeda and P. Data, ACS Appl. Mater. Interfaces, 2021, 13, 2899–2907, DOI:10.1021/acsami.0c17295.
- M. Uji, T. J. B. Zähringer, C. Kerzig and N. Yanai, Angew. Chem., Int. Ed., 2023, 62, e202301506, DOI:10.1002/anie.202301506.
- M. Hayakawa, N. Sunayama, S. I. Takagi, Y. Matsuo, A. Tamaki, S. Yamaguchi, S. Seki and A. Fukazawa, Nat. Commun., 2023, 14, 2741, DOI:10.1038/s41467-023-38300-3.
- J. Cameron, A. L. Kanibolotsky and P. J. Skabara, Adv. Mater., 2023, 2302259, DOI:10.1002/adma.202302259.
- J. F. Joung, M. Han, J. Hwang, M. Jeong, D. H. Choi and S. Park, JACS Au, 2021, 1, 427–438, DOI:10.1021/jacsau.1c00035.
- T. J. Jacobsson, A. Hultqvist, A. García-Fernández, A. Anand, A. Al-Ashouri, A. Hagfeldt, A. Crovetto, A. Abate, A. G. Ricciardulli, A. Vijayan, A. Kulkarni, A. Y. Anderson, B. P. Darwich, B. Yang, B. L. Coles, C. A. R. Perini, C. Rehermann, D. Ramirez, D. Fairen-Jimenez, D. Di Girolamo, D. Jia, E. Avila, E. J. Juarez-Perez, F. Baumann, F. Mathies, G. S. A. González, G. Boschloo, G. Nasti, G. Paramasivam, G. Martínez-Denegri, H. Näsström, H. Michaels, H. Köbler, H. Wu, I. Benesperi, M. I. Dar, I. Bayrak Pehlivan, I. E. Gould, J. N. Vagott, J. Dagar, J. Kettle, J. Yang, J. Li, J. A. Smith, J. Pascual, J. J. Jerónimo-Rendón, J. F. Montoya, J. Correa-Baena, J. Qiu, J. Wang, K. Sveinbjörnsson, K. Hirselandt, K. Dey, K. Frohna, L. Mathies, L. A. Castriotta, M. H. Aldamasy, M. Vasquez-Montoya, M. A. Ruiz-Preciado, M. A. Flatken, M. V. Khenkin, M. Grischek, M. Kedia, M. Saliba, M. Anaya, M. Veldhoen, N. Arora, O. Shargaieva, O. Maus, O. S. Game, O. Yudilevich, P. Fassl, Q. Zhou, R. Betancur, R. Munir, R. Patidar, S. D. Stranks, S. Alam, S. Kar, T. Unold, T. Abzieher, T. Edvinsson, T. W. David, U. W. Paetzold, W. Zia, W. Fu, W. Zuo, V. R. F. Schröder, W. Tress, X. Zhang, Y. Chiang, Z. Iqbal, Z. Xie and E. Unger, Nat. Energy, 2022, 7, 107–115, DOI:10.1038/s41560-021-00941-3.
- Microsoft Bing Chat, asked on 16/Nov/ 2023 Search PubMed.
Footnote |
| † This article is based on the concluding remarks lecture given at the Faraday Discussion meeting on challenges and prospects in organic photonics and electronics, held on 6–8th November 2023. |
| This journal is © The Royal Society of Chemistry 2024 |