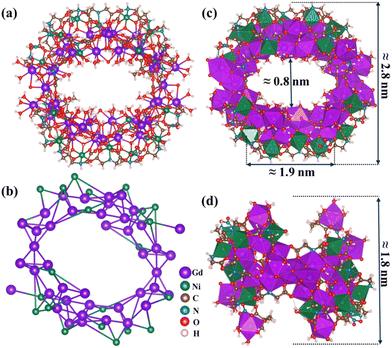
{Gd44Ni22}: a gigantic 3d–4f wheel-like nanoscale cluster with a large magnetocaloric effect†
Zixiu
Lu
 ab,
Zhu
Zhuo
cd,
Wei
Wang
ab,
Zhu
Zhuo
cd,
Wei
Wang
 *cd,
You-Gui
Huang
*cde and
Maochun
Hong
*cd,
You-Gui
Huang
*cde and
Maochun
Hong
 abcd
abcd
aGanjiang Innovation Academy, Chinese Academy of Sciences, Ganzhou 341000, China
bSchool of Rare Earth, University of Science and Technology of China, Ganzhou, China
cCAS Key Laboratory of Design and Assembly of Functional Nanostructures, and Fujian Provincial Key Laboratory of Nanomaterials, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, China. E-mail: wangwei@fjirsm.ac.cn; yghuang@fjirsm.ac.c
dXiamen Key Laboratory of Rare Earth Photoelectric Functional Materials, Xiamen Institute of Rare Earth Materials, Haixi Institutes, Chinese Academy of Sciences, Xiamen, Fujian 361021, China
eFujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou, 350108, China
First published on 13th December 2022
Abstract
Multinuclear 3d–4f nano-clusters, consisting of a large number of metal ions, are interesting both structurally and functionally. The Gd(III) containing clusters, in particular, attract great research attention because of their significant magnetocaloric effect. Here, we report the synthesis of a gigantic 3d–4f wheel-like cluster, {Gd44Ni22}, achieved through self-assembly using a “mixed-ligand” strategy. Magnetic characterization reveals that the {Gd44Ni22} cluster exhibits a large magnetocaloric effect (MCE), with an isothermal magnetic entropy change of 44.9 J kg−1 K−1 at 2.0 K for ΔH = 7 T, which is one of the largest among all of the high-nuclearity Ni–Gd clusters.
Introduction
High-nuclearity 3d–4f metal oxide/hydroxide clusters (M > 25) have attracted extensive research attention because of their fascinating structures and interesting properties.1–10 Among the different properties, a particularly interesting one is the molecular magnetocaloric effect (MCE).11–15 The MCE is a phenomenon that leads to a reversible temperature change when a material is exposed to a changing magnetic field.16 The MCE is safe (without producing greenhouse gases), quiet, cheap, highly durable, and highly efficient (i.e. it requires only a small number of moving parts).17 Currently, the greatest emphasis in the commercial use of MCE is on room temperature cooling (e.g. air conditioning, freezers, etc.). However, the cryogenic application of magnetic cooling materials will be increasingly important with the development of quantum computers, which require ultra-low temperatures.18,19 As a class of highly efficient refrigerants at cryogenic temperatures, molecular magnets are promising materials which exhibit a large MCE.20,21Research reveals that metal oxide/hydroxide clusters displaying large MCEs share certain structural and functional features. These features include the high-spin ground state, large metal/ligand mass ratio (to ensure a high magnetic density), low-lying excited spin states, negligible magnetic anisotropy, and weak magnetic exchange coupling. Following this logic, research efforts on improving the MCE can be roughly divided into three categories. In the first category, 3d-clusters (e.g. {Fe12} and {Mn8}),22–24 with high ground-state spin values, have been studied. However, strong magnetic coupling between transition metal ions results in antiferromagnetic interactions, leading to small MCEs. In the second category, studies have demonstrated larger MCEs in high-nuclearity Gd(III) oxide/hydroxide clusters. This is attributed to the Gd(III) ions with f7 electron configuration, affording multiple low-lying excited spin states and a possible ground state with a large spin value.25 For example, high-nuclearity Gd oxide/hydroxide clusters with large MCEs have been reported recently, including {Gd60} (with the maximum magnetic entropy change (−ΔSm) of 48.0 J kg−1 K−1),12 {Gd104} (−ΔSm = 46.9 J kg−1 K−1),26 {Gd48} (−ΔSm = 43.6 J kg−1 K−1),27etc. Finally, in the third category, Gd(III) ions have been exploited to mitigate the strong magnetic coupling between transition metal ions. By combining 3d metal ions and Gd(III), heterometallic 3d-Gd(III) clusters with small ligands turn out to be most promising for achieving a large MCE.15 Heterometallic 3d-Gd(III) clusters with large MCEs have been reported in recent years, such as {Gd102Ni36} (−ΔSm = 41.3 J kg−1 K−1),28 {Gd96Ni64} (−ΔSm = 42.8 J kg−1 K−1),29 {Gd78Ni64} (−ΔSm = 40.6 J kg−1 K−1),30etc. Among different 3d-Gd(III) clusters, wheel-like clusters are less observed. To date, the only reported 3d-Gd wheel-like structures are {Gd24Cu36}31 and {Gd24Co16},32 both with small −ΔSm of 21.0 J kg−1 K−1 and 26.0 J kg−1 K−1, respectively. It is therefore important to obtain new heterometallic 3d-Gd(III) wheel-like clusters exhibiting a large MCE, to explore the relationship between the cluster structure and magnetocaloric effects.
In this work, a new wheel-like 3d-Gd(III) oxide/hydroxide cluster {Gd44Ni22} (1), with the formula [Gd44Ni22(CO3)16(NO3)4(H2O)58(μ3-OH)76(μ2-OH)6(IDA)28(H2dmpa)2]·(H2O)x (1, x ≈ 118) (H2dmp = 3-hydroxy-2-(hydroxymethyl)-2-methylpropanoic acid, IDA = iminodiacetic acid), is synthesized by using “mixed-ligands” to control the hydrolysis of Gd(III) and Ni(II) ions. The cluster structure and magnetic properties of 1 are investigated in detail to study the structure–property relationship to the magnetocaloric effects.
Results and discussion
Single crystals of the {Gd44Ni22} clusters (Fig. S1†) are obtained through the hydrolysis of Gd(NO3)3·6H2O and Ni(NO3)2·6H2O in the presence of mixed ligands {i.e., iminodiacetic acid (IDA) and 2,2-dimethylol propionic acid (H3dmpa)}. Single-crystal X-ray diffraction (Tables S1–S3†) reveals that {Gd44Ni22} crystallizes in the monoclinic space group P21/n, with the formula [Gd44Ni22(CO3)16(NO3)4(H2O)58(μ3-OH)76(μ2-O)6(IDA)28(H2dmpa)2]·(H2O)x (1, x ≈ 118). The {Gd44Ni22} cluster features a novel giant wheel-like structure with an outer diameter of ∼2.8 nm. The {Gd44Ni22} wheel also possesses an inner cavity, with the dimensions of 0.8 nm and 1.9 nm, respectively, in two perpendicular directions (Fig. 1).By taking a close look, we find that {Gd44Ni22} consists of one wheel-shaped {Gd42Ni22} unit (connected by μ3-OH, CO32− and IDA ligands) and two Gd3+ ions (coordinated by NO3− and H3dmpa ligands). In the structure, the trivalent cations Gd3+ are eight-fold or nine-fold coordinated with O and N atoms, resulting in the [GdO9], [GdO8N], and [GdO8] polyhedra (Fig. S2a, 5b, and 5c†). The Ni2+ ions are all six-coordinated with O and N atoms to form distorted [NiO5N] octahedra (Fig. S2d†). The long distance between two neighboring metal ions (Gd⋯Gd = 3.618–3.971 Å and Gd⋯Ni = 3.452–3.538 Å) likely limits their magnetic interactions.
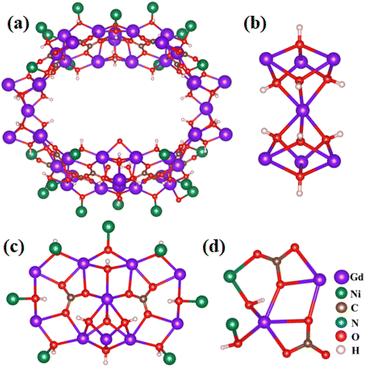
The wheel-shaped {Gd42Ni22} unit (Fig. 2a) consists of three different types of cluster subunits (I, II, and III). Subunit type I, formulated as [Gd7(μ3-OH)8] (Fig. 2b), can be viewed as two cubane-like [Gd4(μ3-OH)4] units that share a common Gd3+ vertex. Type II, formulated as [Gd10Ni7(μ3-OH)12(CO3)2] ({Gd10Ni7}), can be viewed as two CO32− templated five-member rings sharing a Gd3+ vertex while bridged by one additional Gd3+ ion (Fig. 2c). Here, CO32− likely originates from the absorption of atmospheric CO2 by the reaction mixture, as observed by other researchers.12,33 In addition, besides the CO32− ligands, the ten Gd3+ ions in each {Gd10Ni7} unit are connected by twelve hydroxo ligands, with seven Ni2+ ions distributed on the outer edge and connected to Gd3+ ions by μ3-OH. Finally, subunit type III can be described as [Gd2Ni2(μ3-OH)2(CO3)2], with two Gd2+ and two Ni2+ connected by two μ3-OH and two CO32− ligands (Fig. 2d). Two type I subunits, two type II subunits, and four type III subunits are joined together by sixteen CO32−, eighty μ3-OH, and two μ2-O groups, forming the wheel-shaped {Gd42Ni22} component.
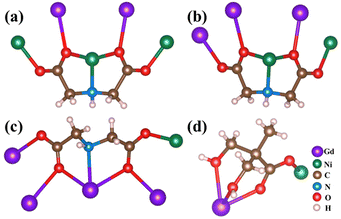
In the structure of {Gd44Ni22}, IDA and H3dmpa have two functions: one is to link the type I, type II, and type III subunits via coordination interactions and the other is to stabilize the wheel-shaped {Gd44Ni22} core. The IDA2− ligand coordinates to the Gd3+ and Ni2+ ions are of three types. The first type coordinates to two Gd3+ ions and three Ni2+ ions in a μ5-ηGd1(O):ηGd1(O):ηNi1(O):ηNi3(O,N,O):ηNi1(O) mode (Fig. 3a). The second type coordinates to three Gd3+ ions and two Ni2+ ions in a μ5-ηGd1(O):ηGd1(O):ηGd1(O):ηNi3(O,N,O):ηNi1(O) mode (Fig. 3b). The third type coordinates to three Gd3+ ions and two Ni2+ ions in a μ5-ηGd1(O):ηGd1(O):ηGd3(O,N,O):ηGd1(O):ηNi1(O) mode (Fig. 3c). Meanwhile, the H3dmpa ligand coordinates to one Gd3+ and one Ni2+ in a μ2-ηGd3(O,O,O):ηNi1(O) mode (Fig. 3d). Structurally, the IDA ligand plays a key role in the formation of a wheel-shaped {Gd42Ni22} unit. Two additional Gd3+ ions attach to the {Gd42Ni22} unit only through H3dmpa ligands, leading to the final {Gd44Ni22} wheel. Interestingly, the packing of {Gd44Ni22} clusters within the lattice results in a nanotube with a one-dimensional channel along the a-axis (Fig. S3†).
It is worth pointing out that high-nuclearity 3d–4f wheel-shaped nanoscale clusters with a large central opening are still underdeveloped. As far as we know, only {CuII36LnIII24} (Ln = Dy and Gd)31 and {CoII16LnIII24} (Ln = Dy and Gd)32 clusters have been reported. {CuII36LnIII24} (Ln = Dy and Gd) consists of two alternating subunits (i.e. cubane-like [Ln4(OH)4] and boat-shaped [Cu6(OH)8(NO3)]). The {CuII36LnIII24} wheel exhibits a diagonal dimension of ∼4.6 nm, a thickness of about ∼1.8 nm, and a central opening with a diameter of ∼0.8 nm. Benzoate is involved in the formation of {CuII36LnIII24}, as the primary linker and the protective ligand. Other wheel-shaped clusters, {CoII16LnIII24} (Ln = Dy and Gd), have been successfully synthesized by adopting pyridyl-functionalized β-diketone as the ligand. The metallo-core of {Co16Ln24} is constructed by a super-square {Ln24} with an octagonal prism {Co16}. The diameter and thickness of the {CoII16LnIII24} cluster are 3.0 nm and 2.0 nm, respectively. Clearly, the {GdIII44NiII22} cluster reported in this work represents a new type of high-nuclearity wheel-shaped cluster, with more metal atoms (44 gadolinium and 22 nickel atoms) than those in the previous examples.
The temperature dependence of direct-current (dc) magnetic susceptibility is characterized on {Gd44Ni22} in an applied magnetic field of 1000 Oe and in the temperature range 2–300 K. The characterization was performed on the polycrystalline powder samples (Fig. S4–S6†). As shown in Fig. S7,† the observed χmT value of 365.9 cm3 K mol−1 (at 300 K) is slightly smaller than the theoretical value of 373.2 cm3 K mol−1 calculated using the Lande formula34,35 based on 22 uncorrelated Ni2+ ions (26.6 cm3 K mol−1 for S = 1 and g = 2.2) and 44 uncorrelated Gd3+ ions (346.7 cm3 K mol−1 for S = 7/2 and g = 2). This result confirms the limited interaction between these metal cations. The data over the temperature range of 2–300 K fit the Curie–Weiss law well, resulting in C = 362.3 cm3 K mol−1 and θ = −5.3 K for the {Gd44Ni22} cluster. The negative θ further confirms the presence of weak antiferromagnetic interactions. This behavior might be ascribed to the weak exchange interactions between the metal ions (Ni⋯Ni, Ni⋯Gd, and Gd⋯Gd) via the bridging ligands.
The generally weak magnetic coupling between Gd3+ and 3d transition metal ions, benefiting from the ability of Gd3+ to mitigate the otherwise strong 3d–3d magnetic exchange, makes the 3d-Gd(III) clusters a valid class of materials for magnetic cooling applications. Here, the presence of a large number of Gd(III) ions in {Gd44Ni22} prompts us to investigate its magnetocaloric effect in the context of developing molecular materials for magnetic cooling.36,37 The field (H) dependence of the magnetization (M) of {Gd44Ni22} at low temperature (2–10 K) is measured (Fig. 4a and Fig. S8†). The M vs. H data show a steady increase in magnetization, reaching 298.3NμB (N is the Avogadro constant and μB is the Bohr magneton) under 7 T at 2 K without achieving saturation (Fig. S8†). This value is slightly lower than the expected value for 66 uncorrelated metal ions (cal. 317.5NμB). This again confirms the weak antiferromagnetic interactions in the cluster. The experimental maximum magnetic entropy change (−ΔSm) is calculated to be 44.9 J kg−1 K−1 at 2 K for ΔH = 7 T using the Maxwell equation, 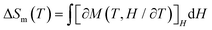 (Fig. 4b). This experimental value is smaller than the theoretical value of 60.0 J kg−1 K−1 (based on the equation Sm = R
(Fig. 4b). This experimental value is smaller than the theoretical value of 60.0 J kg−1 K−1 (based on the equation Sm = R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(2S + 1) for 44 uncorrelated Gd3+ and 22 uncorrelated Ni2+ ions). The smaller experimental value might also be attributed to the presence of weak antiferromagnetic interactions.
ln(2S + 1) for 44 uncorrelated Gd3+ and 22 uncorrelated Ni2+ ions). The smaller experimental value might also be attributed to the presence of weak antiferromagnetic interactions.
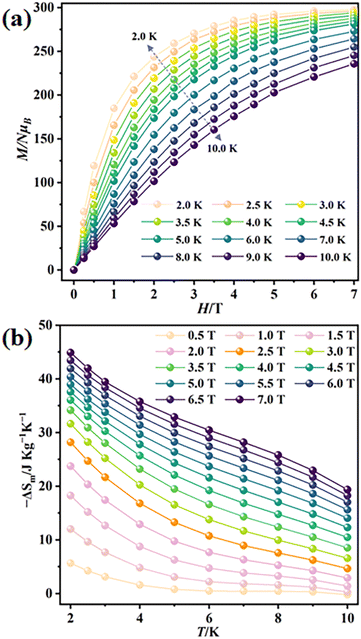 | ||
| Fig. 4 (a) Field dependence of isothermal normalized magnetizations at 2–10 K; (b) plots of experimental magnetic entropy change (−ΔSm) vs. temperature (T) of the wheel-like {Gd44Ni22} cluster. | ||
It is worth noting that the {Gd44Ni22} cluster demonstrates a large MCE, comparable to that of the recently reported high-nucleation cluster {Gd158Co38}38 (−ΔSm = 46.95 J kg−1 K−1 at 2.0 K for ΔH = 7 T). It is also significantly larger than that of other wheel-like 3d-Gd(III) clusters (Table S4†), such as {Gd24Co16}32 (−ΔSm = 26.0 J kg−1 K−1 at 3.8 K for ΔH = 7 T) and {Gd24Cu36}31 (−ΔSm = 21.0 J kg−1 K−1). Meanwhile, the MCE of 1 is also among the largest when compared with the reported homometallic Gd-clusters (Table S4†), demonstrating its great potential for magnetic cooling.
The large MCE of {Gd44Ni22} may be attributed to the following reasons: first, by using ligands with a large number of coordination sites and minor steric hindrances, such as IDA and H3dmpa, we are able to bond and stabilize a large number of metal ions in a relatively compact fashion. This results in a large metal/ligand ratio to ensure a high magnetic density for the large MCE. Second, Gd3+ effectively increases the distance between Ni2+ ions (i.e., to 5.195–5.272 Å), and hence reduces the magnetic coupling between adjacent Ni2+ ions. Still, we notice weak antiferromagnetic couplings in 1 (θ = −5.3 K), indicating that further structural tuning might help to avoid the magnetic interaction and further enhance the MCE of 1.
Conclusion
In summary, a novel wheel-like 3d-Gd cluster, {Gd44Ni22}, is synthesized by adopting the “mixed-ligand” strategy. As a promising magnetic cooling material, {Gd44Ni22} demonstrates a large MCE, with −ΔSm of 44.9 J kg−1 K−1 at 2 K under 7 T. This value is one of the highest among all known high-nuclearity 3d–4f clusters. The large MCE is caused by the high metal/ligand ratio, which leads to high magnetic density. Further studies on tuning the cluster structure to reduce antiferromagnetic interactions and enhance the MCE of 1 are underway and will be reported in our forthcoming contribution.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the NSF of China (21871262 and 21901242), the NSF of Fujian Province (2020J05080), the NSF of Xiamen (3502Z20206080), the Key Research Program of the Chinese Academy of Sciences (ZDRW-CN-2021-3-3), the Youth Innovation Promotion Association CAS (2021302), the Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China (2021ZR110), and the Recruitment Program of Global Youth Experts.References
- T. Liu, E. Diemann, H. Li, A. W. M. Dress and A. Müller, Self-assembly in aqueous solution of wheel-shaped Mo154 oxide clusters into vesicles, Nature, 2003, 426, 59–62 CrossRef CAS PubMed.
- I. Colliard, J. C. Brown, D. B. Fast, A. K. Sockwell, A. E. Hixon and M. Nyman, Snapshots of Ce70 toroid assembly from solids and solution, J. Am. Chem. Soc., 2021, 143, 9612–9621 CrossRef CAS PubMed.
- B. Russell-Webster, J. Lopez-Nieto, K. A. Abboud and G. Christou, Truly monodisperse molecular nanoparticles of cerium dioxide of 2.4 nm dimensions: A {Ce100O167} cluster, Angew. Chem., Int. Ed., 2021, 60, 12591–12596 CrossRef CAS PubMed.
- X. X. Hang, B. Liu, X. F. Zhu, S. T. Wang, H. T. Han, W. P. Liao, Y. L. Liu and C. H. Hu, Discrete {Ni40} coordination cage: A calixarene-based Johnson-Type (J17) hexadecahedron, J. Am. Chem. Soc., 2016, 138, 2969–2972 CrossRef CAS PubMed.
- C. D. Wu, C. Z. Lu, H. H. Zhuang and J. S. Huang, Hydrothermal assembly of a novel three-dimensional framework formed by [GdMo12O42]9− anions and nine coordinated GdIII cations, J. Am. Chem. Soc., 2002, 124, 3836–3837 CrossRef CAS PubMed.
- X. M. Luo, S. Huang, P. Luo, K. Ma, Z. Y. Wang, X. Y. Dong and S. Q. Zang, Snapshots of key intermediates unveiling the growth from silver ions to Ag70 nanoclusters, Chem. Sci., 2022, 13, 11110–11118 RSC.
- Y. F. Bi, X. T. Wang, W. P. Liao, X. F. Wang, X. W. Wang and H. J. Zhang, A {Co32} nanosphere supported by p-tert-butylthiacalix[4]arene, J. Am. Chem. Soc., 2009, 131, 11650–11651 CrossRef CAS PubMed.
- E. G. Ribó, N. L. Bell, W. M. Xuan, J. C. Luo, D. L. Long, T. B. Liu and L. Cronin, Synthesis, assembly, and sizing of neutral, lanthanide substituted molybdenum blue wheels {Mo90Ln10}, J. Am. Chem. Soc., 2020, 142, 17508–17514 CrossRef PubMed.
- S. Kenzler and A. Schnepf, Metalloid gold clusters – Past, current and future aspects, Chem. Sci., 2021, 12, 3116–3129 RSC.
- Z. G. Jiang, W. H. Wu, B. X. Jin, H. M. Zeng, Z. G. Jin and C. H. Zhan, A chloride-doped silver-sulfide cluster [Ag148S26Cl30 (C=CBut)60]6+: Hierarchical assembly, enhanced luminescence and cytotoxicity to cancer cells, Nanoscale, 2022, 14, 1971–1977 RSC.
- L. Qin, Y. Z. Yu, P. Q. Liao, W. Xue, Z. P. Zheng, X. M. Chen and Y. Z. Zheng, A “molecular water pipe”: A giant tubular cluster {Dy72} exhibits fast proton transport and slow magnetic relaxation, Adv. Mater., 2016, 28, 10772–10779 CrossRef CAS PubMed.
- X. M. Luo, Z. B. Hu, Q. F. Lin, W. W. Cheng, J. P. Cao, C. H. Cui, H. Mei, Y. Song and Y. Xu, Exploring the performance improvement of magnetocaloric effect based Gd-exclusive cluster Gd60, J. Am. Chem. Soc., 2018, 140, 11219–11222 CrossRef CAS PubMed.
- H. J. Lun, L. Xu, X. J. Kong, L. S. Long and L. S. Zheng, A high-symmetry double-shell Gd30Co12 cluster exhibiting a large magnetocaloric effect, Inorg. Chem., 2021, 60, 10079–10083 CrossRef CAS PubMed.
- V. Corradini, A. Ghirri, A. Candini, R. Biagi, U. D. Pennino, G. Dotti, E. Otero, F. Choueikani, R. J. Blagg, E. J. L. McInnes and M. Affronte, Magnetic cooling at a single molecule level: a spectroscopic investigation of isolated molecules on a surface, Adv. Mater., 2013, 25, 2816–2820 CrossRef CAS PubMed.
- J. B. Peng, Q. C. Zhang, X. J. Kong, Y. Z. Zheng, Y. P. Ren, L. S. Long, R. B. Huang, L. S. Zheng and Z. P. Zheng, High-nuclearity 3d–4f clusters as enhanced magnetic coolers and molecular magnets, J. Am. Chem. Soc., 2012, 134, 3314–3317 CrossRef CAS PubMed.
- P. Konieczny, W. Sas, D. Czernia, A. Pacanowska, M. Fitta and R. Pełka, Magnetic cooling: A molecular perspective, Dalton Trans., 2022, 51, 12762–12780 RSC.
- X. Y. Zheng, X. J. Kong, Z. P. Zheng, L. S. Long and L. S. Zheng, High-nuclearity lanthanide-containing clusters as potential molecular magnetic coolers, Acc. Chem. Res., 2018, 51, 517–525 CrossRef CAS PubMed.
- E. Warburg, Magnetische untersuchungen, Ann. Phys., 1881, 249, 141–164 CrossRef.
- P. Debye, Einige Bemerkungen zur Magnetisierung bei tiefer Temperatur, Ann. Phys., 1926, 386, 1154–1160 CrossRef.
- W. F. Giauque, A thermodynamic treatment of certain magnetic effects. A proposed method of producing temperatures considerably below 1 absolute, J. Am. Chem. Soc., 1927, 49, 1864–1870 CrossRef CAS.
- O. Tegus, E. Bruck, K. H. J. Buschow and F. R. de Boer, Transition-metal-based magnetic refrigerants for room-temperature applications, Nature, 2002, 415, 150–152 CrossRef CAS PubMed.
- F. Torres, J. M. Hernández, X. Bohigas and J. Tejada, Giant and time-dependent magnetocaloric effect in high-spin molecularmagnets, Appl. Phys. Lett., 2000, 77, 3248–3250 CrossRef CAS.
- T. Lis, Preparation, structure, and magnetic properties of a dodecanuclear mixed-valence manganese carboxylate, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 2042 CrossRef.
- D. Gatteschi, A. Caneschi, L. Pardi and R. Sessoli, Large clusters of metal ions: The transition from molecular to bulk magnets, Science, 1994, 265, 1054 CrossRef CAS PubMed.
- J. W. Sharples, Y. Z. Zheng, F. Tuna, E. J. McInnes and D. Collison, Lanthanide discs chill well and relax slowly, Chem. Commun., 2011, 47, 7650–7652 RSC.
- J. B. Peng, X. J. Kong, Q. C. Zhang, M. Orendac, J. Prokleska, Y. P. Ren, L. S. Long, Z. Zheng and L. S. Zheng, Beauty, symmetry, and magnetocaloric effect four-shell keplerates with 104 lanthanide atoms, J. Am. Chem. Soc., 2014, 136, 17938–17941 CrossRef CAS PubMed.
- F. S. Guo, Y. C. Chen, L. L. Mao, W. Q. Lin, J. D. Leng, R. Tarasenko, M. Orendáč, J. Prokleška, V. Sechovsky and M. L. Tong, Anion-templated assembly and magnetocaloric properties of a nanoscale {Gd38} cage versus a {Gd48} barrel, Chem. – Eur. J., 2013, 19, 14876–14885 CrossRef CAS PubMed.
- W. P. Chen, P. Q. Liao, P. B. Jin, L. Zhang, B. K. Ling, S. C. Wang, Y. T. Chan, X. M. Chen and Y. Z. Zheng, The gigantic {Ni36Gd102} hexagon: A sulfate-templated “star-of-David” for photocatalytic CO2 reduction and magnetic cooling, J. Am. Chem. Soc., 2020, 142, 4663–4670 CrossRef CAS PubMed.
- W. P. Chen, P. Q. Liao, Y. Yu, Z. Zheng, X. M. Chen and Y. Z. Zheng, A mixed–ligand approach for a gigantic and hollow heterometallic cage {Ni64RE96} for gas separation and magnetic cooling applications, Angew. Chem., Int. Ed., 2016, 55, 9375–9379 CrossRef CAS PubMed.
- Q. F. Lin, J. Li, X. M. Luo, C. H. Cui, Y. Song and Y. Xu, Incorporation of silicon–oxygen tetrahedron into novel high-nuclearity nanosized 3d–4f heterometallic clusters, Inorg. Chem., 2018, 57, 4799–4802 CrossRef CAS PubMed.
- J. D. Leng, J. L. Liu and M. L. Tong, Unique nanoscale {CuII 36 LnIII 24}(Ln = Dy and Gd) metallo-rings, Chem. Commun., 2012, 48, 5286–5288 RSC.
- Z. M. Zhang, L. Y. Pan, W. Q. Lin, J. D. Leng, F. S. Guo, Y. C. Chen, J. L. Liu and M. L. Tong, Wheel-shaped nanoscale 3d–4f {Co II 16 Ln III 24} clusters (Ln = Dy and Gd), Chem. Commun., 2013, 49, 8081–8083 RSC.
- X. Y. Zheng, Y. H. Jiang, G. L. Zhuang, D. P. Liu, H. G. Liao, X. J. Kong, L. S. Long and L. S. Zheng, A gigantic molecular wheel of {Gd140}: A new member of the molecular Wheel Family, J. Am. Chem. Soc., 2017, 139, 18178–18181 CrossRef CAS PubMed.
- C. Benelli and D. Gatteschi, Magnetism of lanthanides in molecular materials with transition-metal ions and organic radicals, Chem. Rev., 2002, 102, 2369 CrossRef CAS PubMed.
- O. Kahn, Molecular Magnetism, VCH, New York, 1993 Search PubMed.
- Y. Z. Zheng, G. J. Zhou, Z. Zheng and R. E. P. Winpenny, Molecule-based magnetic coolers, Chem. Soc. Rev., 2014, 43, 1462–1475 RSC.
- J. L. Liu, Y. C. Chen, F. S. Guo and M. L. Tong, Recent advances in the design of magnetic molecules for use as cryogenic magnetic coolants, Coord. Chem. Rev., 2014, 281, 26–49 CrossRef CAS.
- N. F. Li, X. M. Min, J. Wang, J. L. Wang, Y. Song, H. Mei and Y. Xu, Largest 3d–4f 196-nuclear Gd158Co38 clusters with excellent magnetic cooling, Sci. China: Chem., 2022, 65, 1577–1583 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The synthesis; the crystallographic data; the selected bond distance table; the selected bond valence analysis; the decay analysis data; XRD; SEM; IR; TG-DSC. CCDC 2208968 for 1. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qi02294j |
| This journal is © the Partner Organisations 2023 |