Hand-held X-ray fluorescence spectrometry
Analytical Methods Committee AMCTB No. 89
First published on 15th April 2019
Abstract
This Technical Brief describes the evolution of hand-held X-ray fluorescence instrumentation designed for inorganic elemental analysis outside the confines of a laboratory, thereby offering the capability for in situ analysis. As well as providing an overview of the instrumentation, it aims to inform both analysts and less-technically aware users how the technique may be used for a comprehensive assessment of a complex measurement problem and enable on-site decision making.
Introduction
This Technical Brief refers to instrumentation designed for use outside a conventional laboratory for measurements on materials with limited or no sample preparation; rather than in a mobile laboratory transported to a field, industrial or analytical site where conventional sampling and analysis would take place. “Hand-held” instruments, which may also be described as “portable” or “mobile”, allow a wide range of applications not easily achieved with conventional X-ray fluorescence (XRF) instruments.The XRF technique is usually described as offering non-destructive analysis in the sense that the sample introduced into a spectrometer is returned to the user, thereby offering an opportunity for further investigation by other techniques. Hand-held XRF extends the principle of non-destructive analysis to enable larger samples or objects to be measured in position.
Instrumentation, such as the example in Fig. 1, is often calibrated for a specific application and expertise may be needed to interpret results, especially when an in situ surface measurement is used to represent the inaccessible bulk composition of the test item. The immediate availability of analytical results frequently allows the investigator to undertake a comprehensive assessment of a complex problem within a single visit, leading to on-site decision making.
Evolution of hand-held technology
Utilising the energy dispersive configuration coupled with radioactive isotope excitation sources, the early portable instruments found extensive use in measuring lead in paint, workplace monitoring and assessment of contaminated land. Used by academics and some analysts these systems should perhaps have been described as “luggable” rather than “portable” as the batteries, electronic modules and radiation source shielding were large and heavy. As radioisotope sealed sources were used for X-ray excitation detailed planning was required for measurements made outside the confines of a laboratory in order to comply with registration and safety legislation that controls the use of this type of excitation source.1With the introduction of miniature X-ray tubes with less stringent regulatory requirements, lightweight rechargeable batteries, wireless computer technology and global positioning systems (GPS), hand-held XRF became increasingly versatile. More instrument manufacturers realised the potential for in situ analysis with equipment configured and calibrated for specific applications. For example, museum artefacts and works of art could be studied using lightweight hand-held systems rather than a purpose-built configuration of X-ray source, detector and measurement software attached to scaffolding. Hand-held XRF analysers were used by surveyors to prove conformance to building legislation and with the advent of alloy-sorting software, became popular in the scrap metal industry.
With the decreasing size of the instrumentation portable and mobile terminology made way for the more realistic description of hand-held technology. In addition measurements could be made linked to their location with photographs and data transmitted via Wi-Fi or Bluetooth technology transforming a site visit into an on-site decision making process.
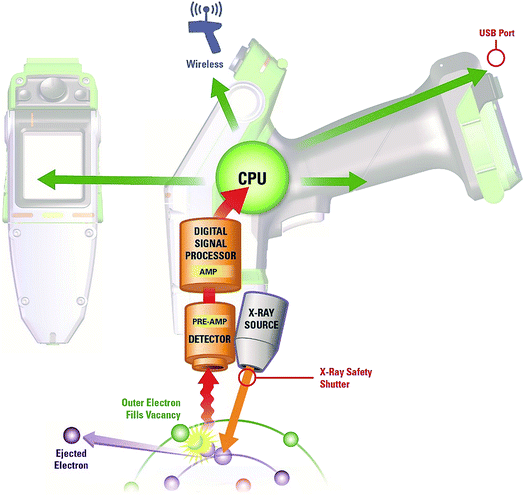
The development of ceramic low-power X-ray tubes (maximum power ∼50 W) and silicon drift detectors (SDDs) or silicon PIN devices coupled with technological development in batteries and microprocessors has led to the fast growing and wide adoption of hand-held analysers, see Fig. 2. The primary X-ray beam from a tube source passes through a beryllium window onto the sample thereby generating characteristic fluorescent spectra from the analytes of interest which are measured by an energy dispersive detector. A signal processing unit either reports measurements as qualitative data or via calibration software as quantitative data for on-screen display. Stored results may also be transmitted via Wi-Fi options to conform to application requirements.
Qualitative and quantitative elemental surface analysis2 is achievable ranging from Mg to U in the periodic table. Measurements on surfaces may be complex, requiring careful consideration of physical matrix effects related to particle size, surface unevenness, sample minerology and moisture content from surface corrosion, oxidation and diffusion effects. Spectral interferences and chemical matrix effects such as absorption and enhancement are corrected by means of fundamental parameter calibrations that offer measurements simultaneously covering ranges from the limit of detection (μg g−1) to the pure material. With qualitative or major element analysis, shorter times of 5–15 seconds are quoted for this non-destructive technique. Hand-held systems are robust and incorporate software for data reporting and evaluation. The so-called “next generation” systems are smaller and lighter in weight (∼1 kg including battery) and offer up to 16 hours of continuous operation. Intuitive, user-friendly software encourages less technically aware operators to use systems calibrated for specific applications. All instruments are designed to satisfy international safety legislation1 with appropriate interlocks and adequate radiation shielding to ensure the user is not exposed to significant levels of either direct or scattered X-rays. Training in the operation of these devices by a wide range of personnel ensures that scientists, engineers, production staff and other professionals find an ever increasing number of applications for this hand-held technology.
Hand-held XRF systems are particularly useful where the instrument is expected to provide measurements to enable decisions in the field, contributing to the resolution of problems rather than simply producing data. Instrumentation may be equipped with wireless and Bluetooth technology to link to a computer or with GPS capability to record measurements with their geographical location. Some instruments also incorporate cameras to record the surface being measured with options for micro or macro-viewing. User-selected collimators allow variable spatial coverage for applications such as spot analysis of jewellery and welds. Dustproof and waterproof systems are also available.
Instrument manufacturers currently offer a wide range of hand-held systems designed for general use or units calibrated for specific applications. Disadvantages compared with laboratory instrumentation exist in areas of quantification, lower sensitivity and the possibility of instrument contamination or damage in the field. The precision of measurements and counting statistics is likely to be inferior to laboratory or larger portable instruments and therefore measurement uncertainty may be larger.
Numerous sources of further information on the instrumentation and its application are available. A monograph2 published in 2008 gave a comprehensive overview of instrumentation and its analytical capabilities, along with limitations in the interpretation of results, sampling considerations and applications where hand-held techniques offer substantial advantages over conventional analytical techniques. In the same year, the Analytical Methods Committee, Royal Society of Chemistry, published Part XXIII of a series of reports3 that tabulated a number of features of analytical instrumentation that should be considered when making comparison between various systems. This document was followed by a Technical Brief,4 designed for a wider readership, which described portable XRF systems and their applications. Progress in hand-held systems is described in the annual Atomic Spectrometry Update (ASU) review of advances in X-ray fluorescence spectrometry and its applications.5
Examples of applications
The wide-ranging capability of hand-held XRF instruments, together with their limitations, can be seen by considering some specific applications reported in the literature.Environment
Environmental assessments on samples of microplastics (n = 924) from two beaches in South West England were analysed6 by hand-held XRF coupled with a ‘small-spot’ facility that collimated the X-ray beam to a diameter of 3 mm. The element content of plastics down to about 1 mm in diameter and 0.1 mm in thickness could be determined rapidly. The contaminants, Cd and Pb were detected in about 7% of the samples analysed with the highest concentrations in red and yellow pellets or fragments and were associated with Se and Cr, respectively. In addition, Br associated with Sb was detected in over 10% of the samples analysed but was mainly found in neutrally-coloured pellets.Glass
Hand-held XRF systems are now extensively used within the glass industry where flat glass producers need to ensure that batch handling machinery and conveyor systems for molten glass are free from nickel which causes contamination. Glass producers incorporate recycled glass (cullet) as a percentage of a furnace batch and require assurance that the cullet is fit for purpose with respect to chemical composition. In the glass container industry, colour changes of the product are commonplace. A glass furnace is designed for continuous operation for some 15 years. During that time the glass colour may be regularly changed to satisfy production demands, for example from white flint to amber or green then back to white. Hand-held XRF systems are predominantly used by engineers to make these production changes as rapidly as possible.Alloy components
For many industries, alloy component reliability, traceability, and safety are of the utmost importance. For example, when testing the material used for small solder joints on aircraft circuit boards or welds in petrochemical refinery pipelines, a 100% positive material identification and verification program can eliminate costly alloy mix-ups, improve product quality and prevent injuries or even loss-of-life.Regulation
Regulatory compliance to European, US and international directives for reducing the impact of harmful elements in consumer goods, on human health and the environment requires the measurement of Pb, Hg, Cr, Sb and other hazardous elements at μg g−1 levels. Suitably calibrated hand-held XRF instruments are able to measure the analytes of interest and report both concentrations and a pass/fail designation.When longer measurement times are required to achieve more sensitivity, a system may be locked into a stand for benchtop operation both in situ and in the laboratory. For example, an application to measure low Sb concentrations in consumer products has been described.7 Concentrations ranged from 60 to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg g−1 but Sb was detected in only 18% of over 800 samples meaning that the concentration in 82% of the samples was <60 μg g−1. The highest concentrations, encountered in white electronic casings, correlated with Br concentrations; a finding that would be consistent with the use of antimony oxides as synergistic flame retardants. Concentrations of Sb were highest (a few%) in plastic components of heat-generating electrical products, but from a health perspective the presence of lower quantities of catalytic Sb in food packaging and small toys was of greatest concern. Co-association of Br and Sb in many products not requiring flame retardant properties suggested that electronic casings are widely recycled.
000 μg g−1 but Sb was detected in only 18% of over 800 samples meaning that the concentration in 82% of the samples was <60 μg g−1. The highest concentrations, encountered in white electronic casings, correlated with Br concentrations; a finding that would be consistent with the use of antimony oxides as synergistic flame retardants. Concentrations of Sb were highest (a few%) in plastic components of heat-generating electrical products, but from a health perspective the presence of lower quantities of catalytic Sb in food packaging and small toys was of greatest concern. Co-association of Br and Sb in many products not requiring flame retardant properties suggested that electronic casings are widely recycled.
Heritage science
Art and archaeometry applications commonly investigate non-uniform samples, determine the provenance of a valued object,8 or obtain elemental data. Original red ochre, original yellow ochre and transformed yellow ochre (nowadays showing a red colour) of wall paintings from Pompeian houses were analysed9 using a hand-held XRF spectrometer. The original red ochre and the transformed yellow ochre could be differentiated. Principle component analysis of the multivariate data showed that the As content could be used to distinguish between both red-coloured ochres. The element composition and the conclusions drawn from the in situ analysis were confirmed by laboratory measurements.Conclusions
Hand-held XRF instruments have become indispensable for a wide range of commercial, regulatory, health, environmental, consumer safety and other applications. Modern instrumentation has developed a degree of sophistication which has enabled a wider application of XRF technology than is possible by conventional laboratory techniques. In particular, it often facilitates rapid, on site decision making by professionals in their field without the need for specialist analytical expertise.The Analytical Methods Committee (AMC) does not endorse any particular instrument manufacturer but thanks Thermo Fisher Scientific for their permission to use their image in this Technical Brief.
Margaret West (XRF Consultant)
This Technical Brief was prepared for the AMC, with contributions from members of the AMC Instrumental Analysis Expert Working Group, and approved on 13 March 2019.
Further reading
- Ionising Radiation Regulations 2017 (IRR17), Approved Code of Practice and guidance L121, The Stationery Office, 2018, ISBN 9780717666621 Search PubMed.
- Portable X-ray fluorescence spectrometry, ed. P. J. Potts and M. West, RSC Publishing, Cambridge, 2008, ISBN 978-0-85404-552-5 Search PubMed.
- Evaluation of Analytical Instrumentation - Part XXIII Instrumentation for Portable X-ray Fluorescence Spectrometry published in ACQUAL, 2008, 13 (8), 453–464, http://link.springer.com/article/10.1007/s00769-008-0358-x.
- AMC Technical Brief – Portable X-ray fluorescence analysis, No. 41, June 2009.
- C. Vanhoof, J. R. Bacon, A. T. Ellis, L. Vincze and P. Wobrauschek, 2018 atomic spectrometry update–a review of advances in X-ray fluorescence spectrometry and its special applications, J. Anal. At. Spectrom., 2018, 33, 1413–1431 RSC.
- A. Massos and A. Turner, Cadmium, lead and bromine in beached microplastics, Environ. Pollut., 2017, 227, 139–145 CrossRef CAS PubMed.
- A. Turner and M. Filella, Field-portable-XRF reveals the ubiquity of antimony in plastic consumer products, Sci. Total Environ., 2017, 584, 982–989 CrossRef PubMed.
- AMC Technical Brief – X-ray fluorescence (XRF) analysis of porcelain: Background paper, No. 77, March 2017.
- I. Marcaida, M. Maguregui, S. F. O. de Vallejuelo, H. Morillas, N. Prieto-Taboada, M. Veneranda, K. Castro and J. M. Madariaga, In situ X-ray fluorescence-based method to differentiate among red ochre pigments and yellow ochre pigments thermally transformed to red pigments of wall paintings from Pompeii, Anal. Bioanal. Chem., 2017, 409(15), 3853–3860 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |