Thermal evolution of structures and conductivity of Pr-substituted BaZr0.7Ce0.2Y0.1O3−δ: potential cathode components for protonic ceramic fuel cells
Abstract
A complete solid solution forms between the perovskite proton conductor BaZr0.7Ce0.2Y0.1O3−δ (BZCY72) and BaPr0.9Y0.1O3−δ (BPY) on synthesis by the Pechini method and high-temperature annealing. Phase fields of selected members of the Ba(Zr0.7Ce0.2)1−(x/0.9)PrxY0.1O3−δ series were studied as a function of composition and temperature by high-resolution neutron powder diffraction revealing symmetry changes in the sequence Pnma → Imma → 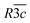 → Pm
→ Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. Higher symmetry is favoured for low Pr contents and high temperatures, as consideration of tolerance factor suggests. A volume contraction, ascribed to dehydration, is observed by synchrotron X-ray diffraction on heating in air for lower x. Magnetic measurements and structural data support the presence of Pr in the IV valence state on the perovskite B site. Thermogravimetric analysis in CO2 to ∼1253 K indicates better chemical stability for x ≤ 0.445, whereas decomposition occurred for higher x. Electrical conductivity increases by over two orders of magnitude in dry air at lower temperature from x = 0.225 to 0.675; total conductivity reaches a value of 0.4 S cm−1 at 1173 K for x = 0.675. The series exhibits electron–hole transport with a positive pO2 dependence which increases with temperature, consistent with participation of oxygen vacancies in charge compensation of the Y3+ acceptor dopant. The activation energy for thermally activated hole hopping in air in the range 523–773 K decreases from ∼1 eV for BZCY72 to ∼0.4 eV for x = 0.675. Conductivity is generally lower in humidified N2 and air (pH2O ≈ 0.023 atm) than the corresponding dry atmospheres, consistent with consumption of holes by less mobile protonic species; however for x ≤ 0.225 the lower concentration of electron holes concomitant with higher oxygen-vacancy content in N2 results in slightly higher conductivity in wet conditions due to hydration of vacancies.
m. Higher symmetry is favoured for low Pr contents and high temperatures, as consideration of tolerance factor suggests. A volume contraction, ascribed to dehydration, is observed by synchrotron X-ray diffraction on heating in air for lower x. Magnetic measurements and structural data support the presence of Pr in the IV valence state on the perovskite B site. Thermogravimetric analysis in CO2 to ∼1253 K indicates better chemical stability for x ≤ 0.445, whereas decomposition occurred for higher x. Electrical conductivity increases by over two orders of magnitude in dry air at lower temperature from x = 0.225 to 0.675; total conductivity reaches a value of 0.4 S cm−1 at 1173 K for x = 0.675. The series exhibits electron–hole transport with a positive pO2 dependence which increases with temperature, consistent with participation of oxygen vacancies in charge compensation of the Y3+ acceptor dopant. The activation energy for thermally activated hole hopping in air in the range 523–773 K decreases from ∼1 eV for BZCY72 to ∼0.4 eV for x = 0.675. Conductivity is generally lower in humidified N2 and air (pH2O ≈ 0.023 atm) than the corresponding dry atmospheres, consistent with consumption of holes by less mobile protonic species; however for x ≤ 0.225 the lower concentration of electron holes concomitant with higher oxygen-vacancy content in N2 results in slightly higher conductivity in wet conditions due to hydration of vacancies.

- This article is part of the themed collection: Advances in Solid State Chemistry and its Applications


 Please wait while we load your content...
Please wait while we load your content...