Analytical pyrolysis in cultural heritage
Analytical Methods Committee, AMCTB No. 85
First published on 20th November 2018
Abstract
Analytical pyrolysis (Py), especially when coupled with gas chromatography and mass spectrometry (Py-GC-MS), is a powerful technique for the characterisation and identification of organic materials used in artwork. The thermal degradation of macromolecules (for example, resins, lacquers, proteins, polysaccharides, oils, modern synthetic polymers, etc.) using heat (thermal energy) generates smaller molecules (pyrolysis products) which are easier to identify and study. Some of these pyrolysis products are molecular markers allowing the identification of a specific material despite the sample’s complexity. A micro-sample (50–100 μg) is destroyed during the analysis but the lack of sample preparation makes Py-GC-MS a very attractive technique with a much reduced analytical time and cost compared to other chromatographic methods.
Introduction
Pyrolysis is the decomposition of a material at high temperature in the absence of oxygen. Its use in combination with gas chromatography first appeared in 1960. In the 1970s and 1980s it was used to characterise natural and synthetic polymers of industrial importance. The late 1990s saw the first applications to artwork materials. Initially, macromolecules such as polysaccharides and proteins were the main targets but resins, waxes, lacquer, wood and synthetic polymers were also studied soon after. In the last twenty years there has been a significant increase in the application of analytical pyrolysis to the characterisation of organic materials used in artwork. It is now used as a screening technique to study complex mixtures and to investigate particular properties of natural and synthetic polymers, such as their chemical degradation.What is analytical pyrolysis good for?
In the cultural heritage field, the main use of Py-GC-MS is the identification of organic materials in composite samples. For example:Paint samples
Binding media (oils, proteins and gums) and coating materials, such as varnishes (resins and waxes), can be identified and sometimes differentiated.Lacquer
Different types of both Asian and European lacquer can be characterised.Modern materials
Synthetic formulations, such as alkyd, acryl and vinyl resins, other synthetic polymers (plastics) and many synthetic organic pigments, are also identified.Fibres
Cellulose-based fibres (cotton and flax) can be distinguished from proteinaceous fibres (silk and keratin).Lignocellulosic materials
Wood and wood products can be identified, although not at the species level.Degradation of materials
In addition to the identification of materials, analytical pyrolysis is also useful for the study of their degradation. Most of such applications study synthetic polymers and lignocellulosic materials, in particular wood.How does it work?
Pyrolysis is a process in which heat (thermal energy) is used to break the molecular bonds in a large molecule (a macromolecule like a protein or a polymer like a plastic) and to form the so-called pyrolysis products. The idea behind this is to reduce the complexity of the original molecule and study some of its features by looking at the simpler and smaller pyrolysis products (Fig. 1).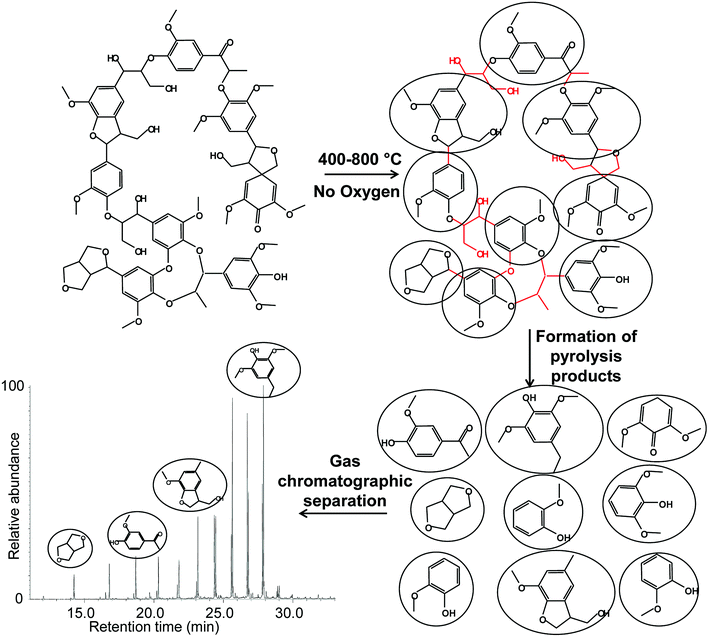 | ||
| Fig. 1 Simplified and hypothetical scheme of the pyrolysis process of a general macromolecule, showing the formation and gas chromatographic separation of the pyrolysis products. | ||
Temperatures between 400 °C and 800 °C are generally used. To avoid combustion the entire process occurs in the absence of oxygen.
Types of pyrolyser and instrumental configurations
Two main types of pyrolyser are used in analytical work.Filament pyrolysers are equipped with a metal coil at the end of a probe. A quartz tube containing the sample can be inserted in it. The probe is introduced into a pyrolysis chamber and is electrically heated to the desired temperature (Fig. 2a and b).
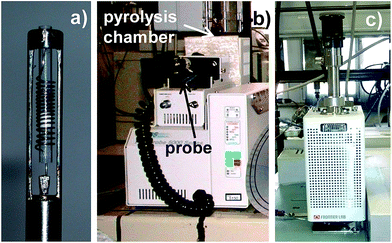 | ||
| Fig. 2 (a) The probe of a filament pyrolyser, showing the coil and the quartz tube; (b) the same filament pyrolyser with the probe inserted into the pyrolysis chamber; (c) a micro-furnace pyrolyser. | ||
Furnace or micro-furnace pyrolysers have a pre-heated pyrolysis chamber. The sample is loaded at the top of the chamber, usually using a stainless steel cup, and then dropped inside by a releasing mechanism. The transfer of the sample into the cup and its weighing are easier than the process involving a quartz pyrolysis tube and better reproducibility has been reported (Fig. 2c).
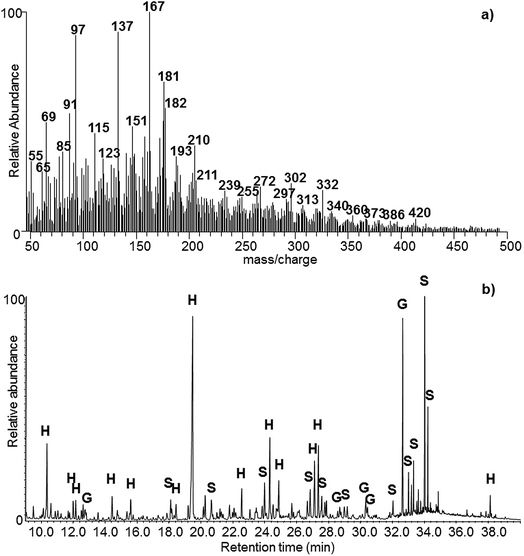
Pyrolysers can be directly coupled with a detector, usually a mass spectrometer. This configuration is called Py-MS or DE-MS (direct exposure mass spectrometry). Gas chromatographic separation is often interposed between the pyrolyser and the mass spectrometer (Py-GC-MS). In the absence of a chromatographic separation, the result is an overall mass spectrum of all the pyrolysis products (Fig. 3a). However, certain prominent masses can stand out and be used to identify molecules, or chemometric methods can be applied to highlight information. In Py-GC-MS all pyrolysis products are separated in the GC column and can therefore be identified one by one, making it easier to identify markers and interpret data (Fig. 3b).
Recent instrumental developments have led to other pyrolysis modes. It is possible to program the pyrolysis temperature as a ramp rather than a single temperature. This produces a slow pyrolysis of the sample at increasing temperatures and the pyrolysis products, directly transferred to the mass spectrometer, can be studied as a function of the temperature. This mode is referred to as evolved gas analysis mass spectrometry (EGA-MS). Alternatively, the same sample can undergo more than one pyrolysis shot at different temperatures and GC-MS can follow each shot. If two temperatures are chosen, the technique takes the name of ‘double-shot Py-GC-MS’. If more than two temperatures are chosen, the technique is referred to as ‘multi-shot Py-GC-MS’.
Advantages and limitations
The main advantage of analytical pyrolysis over other chromatographic techniques is that no sample preparation is required: the sample is analysed in its solid state without undergoing any wet chemical treatment. This drastically reduces the analysis time and the costs of solvents and equipment used in sample pre-treatment. Even in the case of complex mixtures no prior chemical separation is necessary as the specific molecular markers are produced in the pyrolysis process and are sufficient to identify the materials present in the sample.Moreover, some natural and synthetic polymers do not respond well to chemical treatments: the Py-GC-MS method is clearly invaluable in obtaining detailed molecular information in these cases.
Among the limitations of the technique there is the requirement of the pyrolysis products to be sufficiently volatile to undergo gas chromatography. This problem can be overcome by the use of derivatising agents such as tetramethylammonium hydroxide (TMAH), for the process called methylation, and hexamethyl-disilazane (HMDS) for silylation. These agents are added in situ directly in the sample holder together with the solid sample. Their function is to substitute polar OH groups thereby reducing the polarity of the pyrolysis products and enhancing their volatility.
Another limitation is that Py-GC-MS is not a quantitative technique, so while materials can be qualitatively identified, it is difficult to assess how much of each is present in a mixture. This is because each material has its own pyrolysis yield, an intrinsic property that is difficult to predict, especially because other materials and inorganic compounds that may be present can affect the pyrolytic pathways and thus the pyrolysis yield. In addition, one material can generate so many pyrolysis products that it is virtually impossible to obtain a calibration curve for each. Nevertheless, pyrolysis data can be used in a semi-quantitative way when the chromatographic areas of pyrolysis products are integrated, summed and expressed as percentages. Such percentages are not necessarily related to absolute concentrations, but can give information on the degradation of the material itself. These comparisons are correct only if the same experimental conditions are used within a single laboratory.
Finally, the interpretation of a pyrogram can be a challenging and time-consuming process, requiring a high level of expertise in chromatographic analysis and in the interpretation of mass spectra.
Case study: Py-GC-MS analysis of archaeological wood
Wood is a complex natural material. It is mainly composed of three interlinked biopolymers, cellulose, hemicellulose and lignin. Archaeological wood is found in particular burial conditions such as waterlogged, very dry or frozen ground. Before applying any conservation treatment, usually needed to guarantee the structural stability of wood, it is important to evaluate its state of chemical degradation. This can be estimated by measuring the residual cellulose, hemicellulose and lignin in the sample and comparing the results with those from a sample of sound reference wood of the same species.The pyrogram of a wood sample mainly contains pyrolysis products from the three polymers. The first step is therefore to identify the products and assign them to the right polymer. Cellulose and hemicellulose produce the same pyrolysis products and cannot be distinguished: the sum of cellulose and hemicellulose is thus called holocellulose. By contrast, p-hydroxyphenyl-lignin, guaiacyl-lignin and syringyl-lignin can be distinguished through their pyrolysis products. As p-hydroxyphenyl-lignin is predominant in grasses, guaiacyl-lignin in conifers (softwoods) and syringyl-lignin in broad-leaved trees (hardwoods), these types of plants can be distinguished, though the exact plant species cannot be identified by pyrolysis and anatomical observations are still the best way to obtain this information.
Once holocellulose (H) and lignin (L) pyrolysis products are identified, initial information about the degradation state of the wood is given by the so-called H/L ratio. Percentage chromatographic areas of each pyrolysis product are calculated and those of the pyrolysis products from holocellulose and lignin are summed separately. The ratio between these two values provides the pyrolytic H/L ratio. When this ratio is compared between a sound reference wood (H/Lref) and an archaeological wood (H/Larch) of the same species, an estimation of the prevalent loss of holocellulose or lignin in the archaeological sample is obtained. In particular:
- if H/Larch < H/Lref, holocellulose is mostly lost;
- if H/Larch > H/Lref, lignin is mostly lost.
However, the H/L ratio has some limitations, as it does not provide information about the degradation of individual wood components (holocellulose and lignin). To provide this information, the holocellulose and lignin pyrolysis products can be further categorised according to their molecular structure and pyrolytic formation, as exemplified in Fig. 4.
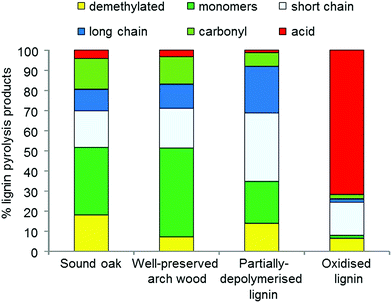 | ||
| Fig. 4 An example of the distribution of lignin pyrolysis products divided into categories in a sound wood sample and in archaeological samples showing different preservation conditions. | ||
Lignin oxidation, depolymerisation and demethylation can be seen, as well as cellulose depolymerisation. Such information can be related to a particular cause of degradation and inform about the best treatments to be used to preserve the wood.
Further reading
- S. C. Moldoveanu, Pyrolysis of organic molecules with applications to health and environment, Elsevier, Oxford (UK), 2010 Search PubMed.
- I. Bonaduce and A. Andreotti, Py-GC/MS of Organic Paint Binders in Organic Mass Spectrometry in Art and Archeology, ed. M. P. Colombini and F. Modugno, Wiley, Chichester (UK), 2009, pp. 303–326, DOI:10.1002/9780470741917.ch11.
- I. Degano, F. Modugno, I. Bonaduce, E. Ribechini and M. P. Colombini, Recent Advances in Analytical Pyrolysis to Investigate Organic Materials in Heritage Science, Angew. Chem., Int. Ed., 2018, 57, 7313–7323, DOI:10.1002/anie.201713404.
- A. P. Pinder, I. Panter, G. D. Abbott and B. J. Keely, Deterioration of the Hanson Logboat: chemical and imaging assessment with removal of polyethylene glycol conserving agent, Sci. Rep., 2017, 7, 13697, DOI:10.1038/s41598-017-14057-w.
- S. Wei, V. Pintus and M. Schreiner, Photochemical degradation study of polyvinyl acetate paints used in artworks by Py–GC/MS, J. Anal. Appl. Pyrolysis, 2012, 97, 158–163, DOI:10.1016/j.jaap.2012.05.004.
- D. Tamburini, J. J. Łucejko, E. Ribechini and M. P. Colombini, New markers of natural and anthropogenic chemical alteration of archaeological lignin revealed by in situ pyrolysis/silylation-gas chromatography-mass spectrometry, J. Anal. Appl. Pyrolysis, 2016, 118, 249–258, DOI:10.1016/j.jaap.2016.02.008.
This Technical Brief was prepared by the Heritage Science Subcommittee and approved by the Analytical Methods Committee on 05/10/2018.
| This journal is © The Royal Society of Chemistry 2018 |