Open Access Article
Open Access ArticleA metal-catalyzed enyne-cyclization step for the synthesis of bi- and tricyclic scaffolds amenable to molecular library production†
Peng
Wu‡
a,
Michael Åxman
Petersen‡
a,
A. Emil
Cohrt‡
a,
Rico
Petersen
a,
Rémy
Morgentin
b,
Hugues
Lemoine
b,
Carine
Roche
b,
Anthony
Willaume
b,
Mads H.
Clausen
*ac and
Thomas E.
Nielsen
*ad
aDepartment of Chemistry, Technical University of Denmark, DK-2800, Kgs. Lyngby, Denmark. E-mail: ten@sund.ku.dk
bEDELRIS, 115 Avenue Lacassagne, 69003 Lyon, France
cCenter for Nanomedicine and Theranostics, Technical University of Denmark, DK-2800, Kgs. Lyngby, Denmark
dSingapore Centre on Environmental Life Science Engineering, Nanyang Technological University, 637551, Singapore
First published on 24th June 2016
Abstract
A facile metal-catalyzed diversification step for the synthesis of novel bi- and tricyclic scaffolds from enyne substrates is reported in this study. From a single starting material, topologically diverse scaffolds for library synthesis can be generated and decorated in a few steps. The methodology was used to produce a library of 490 compounds within the European Lead Factory (ELF) Consortium.
Synthetically tractable scaffolds populating unexplored areas of chemical space are highly sought-after in early-stage drug discovery, particularly in the hunt for hits against challenging macromolecular targets.1 It has been suggested that small-molecule candidates containing fewer aromatic rings and a high fraction of sp3-hybridized carbon atoms (Fsp3) exhibit improved molecular solubility and reduced attrition rate.2,3 On the other hand, aromatic rings are likely to contribute to high binding affinities and potency due to their hydrophobic properties and rigid structures, and compelling correlations between the Fsp3 value and the hit rate have been elucidated in fragment-based drug discovery analysis.4 Most FDA-approved small-molecule drugs that are administered orally, including >90% of approved kinase inhibitors,5,6 have an aromatic ring count value between two and four.7 Thus, the delicate balance between physicochemical properties and pharmacological potency makes the design of optimal scaffolds a daunting task in drug discovery.
The synthesis of structurally complex and diverse scaffolds from easily accessible building blocks with multiple functional groups is highly sought-after in the current field of library synthesis.8 In our group, several sp3-rich scaffolds containing multiple chiral centers have been utilized for the production of molecular libraries of up to 500 compounds.9–13 Following our previous work within diversity-oriented synthesis, we herein report a facile and scalable metal-catalyzed cyclization approach for the synthesis of bi- and tricyclic scaffolds from enyne substrates. For proof-of-principle, one of the scaffolds was used as a core template for the production of a library containing 490 compounds in the ELF Consortium.14–16
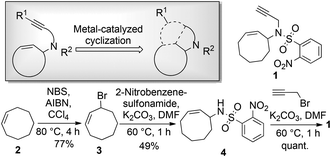
Enyne substrates have been studied in various metal-catalyzed reactions, such as the well-investigated enyne metathesis and various cycloisomerization reactions.17–23 The nosyl-protected enyne compound 1 was designed for use in various cyclization reactions and readily obtained through a straightforward route starting from cyclooctene (Scheme 1). To introduce appendage diversity from any resulting scaffold, a reactive alkenyl halide handle would be highly desirable, e.g. for smooth metal-catalyzed cross-coupling reactions. Introduction of a halide prior to a metal-catalyzed enyne-cyclization would be advantageous, particularly if the halide could be retained during several scaffold-generating diversification steps, such as the application of iodoalkynes in cylcoisomerization reactions.19,20,23 A substantial challenge, however, would be constituted by the inherent reactivity of the halide functionality.
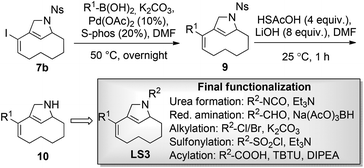
In this context, we decided to synthesize iodoenyne 5 and subject this substrate to metal-catalyzed cyclization reactions that allow the formation of new scaffolds without compromising the halide handle. In a successful embodiment, metal-catalyzed cyclization could then be followed by appendage diversifying cross-coupling reactions, ideally providing substituted sp3-rich scaffolds (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value <3) of low molecular weight (<300 Da) (Scheme 2).§
P value <3) of low molecular weight (<300 Da) (Scheme 2).§
 | ||
| Scheme 2 Metal-catalyzed cyclization of enynes for the synthesis of diverse scaffolds for library synthesis. | ||
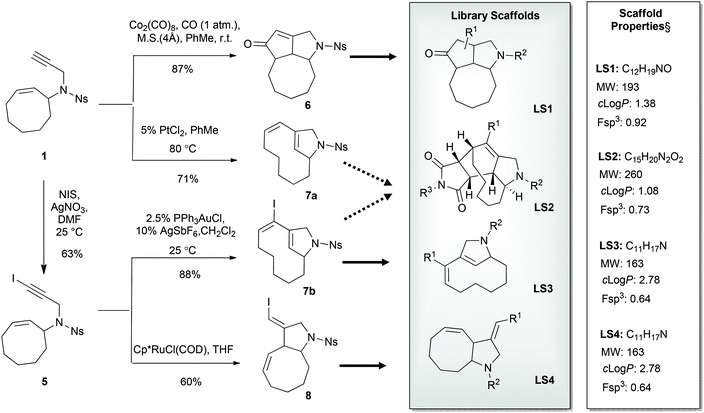
When iodoenyne 5 was subjected to ruthenium-based metathesis conditions, only complex reaction mixtures were obtained. Whereas the platinum-catalyzed metathesis reaction of 1 gave the expected product 7a using PtCl2,24 the iodinated analogue 5 did not undergo metathesis under these conditions. In further synthetic studies on compound 5, it was discovered that the bicyclic product 7b could be synthesized in excellent yield using PPh3AuCl/AgSbF6 as the catalytic system.25 During screening of different transition metal catalysts and reagents,26 it was also shown that compound 1 could be cyclized to the tricyclic compound 6 in a Pauson–Khand reaction using carbon monoxide in the presence of Co2(CO)8. Unfortunately, the iodoenyne 5 could not be converted to the corresponding tricyclic compound under the same conditions. In addition, compound 5 could be smoothly rearranged to the bicyclic compound 8 using the ruthenium-based catalyst precursor Cp*RuCl(COD).27,28
Attempts were made to form the tetracyclic scaffold LS2 from 7a and 7bvia maleimide Diels–Alder reactions. With a high Fsp3 value and a substantial number of chiral centers and appendage diversification handles, LS2 represents an interesting scaffold for library synthesis. Presumably, the twisted nature of the diene moiety of 7 makes the formation of Diels–Alder products impossible irrespective of the dienophile. It was therefore decided to bring scaffold LS3 forward as a library core structure based on compound 7b, which has two potential diversification handles. The R1 group could selectively be varied using different boronic acids in Suzuki cross-coupling reactions. The obtained N-nosyl compounds 9 were deprotected to give amines 10, which were subjected to a final functionalization step to introduce R2 groups of diverse nature (Scheme 3). Numerous compounds were prepared during library production. For example, compound 10 was treated with aryl isocyanates in the presence of triethylamine to give ureas 11a and 11b; reductive amination with aryl aldehydes afforded compounds 12a, 12b, and 12c; alkylation of compound 10 with methyl chloroacetate led to compound 13; sulfonylation with sulfonyl chlorides gave compounds 14a and 14b; and acylation of compound 10 under TBTU-mediated coupling conditions afforded compounds 15a–d (Scheme 4). The above-validated synthetic route was scaled up to 160 mmol scale for the intermediate core scaffold 7b (75 g) from 550 mmol of cyclooctene 2 (70 g) in the indicated 5 steps. Functionalization of the R1 group of 7b through Suzuki-coupling, followed by nosyl-deprotection, and a final amine-substitution step yielded a library of 490 compounds for the European Lead Factory Consortium.¶
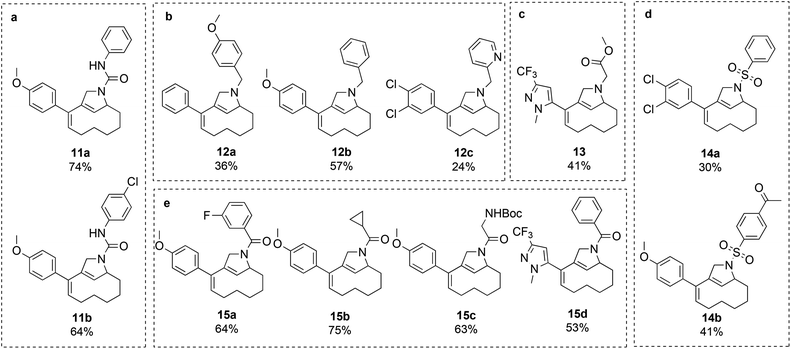 | ||
| Scheme 4 Validated compounds for the library establishment based on the LS3 scaffold. (a) Urea formation; (b) reductive amination; (c) alkylation; (d) sulfonylation; (e) acylation. | ||
A total of 654 new screening compounds, most of which are compliant with the Lipinski's Rule of Five (Fig. 1), were selected amongst the enumerated library and synthesized in 9 production campaigns with an overall success rate of 75% after purification under the optimized conditions.
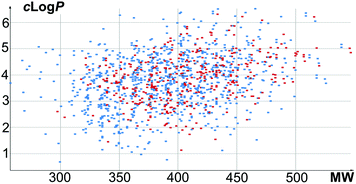 | ||
Fig. 1 Physical chemical property analysis of library compounds (blue spots: 903 enumerated compounds; red spots: 490 validated compounds), clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P vs. MW. P vs. MW. | ||
In this study, we have demonstrated the feasibility of metal-catalyzed cyclizations for the synthesis of structurally diverse bi- and tricyclic scaffolds from a readily available iodoenyne. From a single starting material, topologically diverse scaffolds for library synthesis can be generated and decorated in a few steps. Scaffold LS3 with two appendage functionalization handles was selected as a proof-of-concept for the synthesis of a library of 490 compounds, which will be screened against a range of biological targets within the ELF Consortium. Further library productions based on other scaffolds from this study will be reported in due course.
Acknowledgement is made to the Innovative Medicines Initiative Grant (115489, FP7/2007–2013), the Lundbeck Foundation (R141-2013-13835), and the Technical University of Denmark. We also thank Jennifer Mehlen, Julie Raud, Guillaume Ranty, and Caroline Gurcel at Edelris for assistance in the purification of the final library compounds.
Notes and references
- C. M. Marson, Chem. Soc. Rev., 2011, 40, 5514 RSC.
- F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752 CrossRef CAS PubMed.
- T. J. Ritchie and S. J. F. Macdonald, Drug Discovery Today, 2009, 14, 1011 CrossRef CAS PubMed.
- M. Hansson, J. Pemberton, O. Engkvist, I. Feierberg, L. Brive, P. Jarvis, L. Zander-Balderud and H. Chen, J. Biomol. Screening, 2014, 19, 727 CrossRef PubMed.
- P. Wu, T. E. Nielsen and M. H. Clausen, Drug Discovery Today, 2016, 21, 5 CrossRef CAS PubMed.
- P. Wu, T. E. Nielsen and M. H. Clausen, Trends Pharmacol. Sci., 2015, 36, 422 CrossRef CAS PubMed.
- R. D. Taylor, M. MacCoss and A. D. G. Lawson, J. Med. Chem., 2014, 57, 5845 CrossRef CAS PubMed.
- D. J. Foley, R. G. Doveston, I. Churcher, A. Nelson and S. P. Marsden, Chem. Commun., 2015, 51, 11174 RSC.
- R. Petersen, A. E. Cohrt, M. Å. Petersen, P. Wu, M. H. Clausen and T. E. Nielsen, Bioorg. Med. Chem., 2015, 23, 2646 CrossRef CAS PubMed.
- M. Å. Petersen, M. A. Mortensen, A. E. Cohrt, R. Petersen, P. Wu, N. Fleury-Brégeot, R. Morgentin, C. Lardy, T. E. Nielsen and M. H. Clausen, Bioorg. Med. Chem., 2015, 23, 2695 CrossRef CAS PubMed.
- P. Wu, M. Å. Petersen, R. Petersen, M. O. Rasmussen, K. Bonnet, T. E. Nielsen and M. H. Clausen, Eur. J. Org. Chem., 2015, 5633 CrossRef CAS.
- P. Wu, M. Å. Petersen, A. E. Cohrt, R. Petersen, M. H. Clausen and T. E. Nielsen, Eur. J. Org. Chem., 2015, 2346 CrossRef CAS.
- P. Wu, M. A. Petersen, R. Petersen, T. Flagstad, R. Guilleux, M. Ohsten, R. Morgentin, T. E. Nielsen and M. H. Clausen, RSC Adv., 2016, 6, 46654 RSC.
- A. Mullard, Nat. Rev. Drug Discovery, 2013, 12, 173 CrossRef PubMed.
- J. Besnard, P. S. Jones, A. L. Hopkins and A. D. Pannifer, Drug Discovery Today, 2015, 20, 181 CrossRef CAS PubMed.
- A. Karawajczyk, F. Giordanetto, J. Benningshof, D. Hamza, T. Kalliokoski, K. Pouwer, R. Morgentin, A. Nelson, G. Müller, A. Piechot and D. Tzalis, Drug Discovery Today, 2015, 20, 1310 CrossRef CAS PubMed.
- H. Villar, M. Frings and C. Bolm, Chem. Soc. Rev., 2007, 36, 55 RSC.
- V. Michelet, P. Y. Toullec and J.-P. Genêt, Angew. Chem., Int. Ed., 2008, 47, 4268 CrossRef CAS PubMed.
- P. Morán-Poladura, E. Rubio and J. M. González, Angew. Chem., Int. Ed., 2015, 54, 3052 CrossRef PubMed.
- A. Furstner, A. Schlecker and C. W. Lehmann, Chem. Commun., 2007, 4277 RSC.
- B. M. Trost, A. C. Gutierrez and E. M. Ferreira, J. Am. Chem. Soc., 2010, 132, 9206 CrossRef CAS PubMed.
- P. R. Walker, C. D. Campbell, A. Suleman, G. Carr and E. A. Anderson, Angew. Chem., Int. Ed., 2013, 52, 9139 CrossRef CAS PubMed.
- P. Nösel, T. Lauterbach, M. Rudolph, F. Rominger and A. S. K. Hashmi, Chem. – Eur. J., 2013, 19, 8634 CrossRef PubMed.
- A. Fürstner, H. Szillat and F. Stelzer, J. Am. Chem. Soc., 2000, 122, 6785 CrossRef.
- C. Obradors and A. M. Echavarren, Acc. Chem. Res., 2014, 47, 902 CrossRef CAS PubMed.
- H. Pellissier and H. Clavier, Chem. Rev., 2014, 114, 2775 CrossRef CAS PubMed.
- N. Saito, D. Tanaka, M. Mori and Y. Sato, Chem. Rec., 2011, 11, 186 CrossRef CAS PubMed.
- J. Le Paih, D. Cuervo Rodríguez, S. Dérien and P. H. Dixneuf, Synlett, 2000, 95 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: General methods, experimental procedures, characterization data, and 1H and 13C NMR spectra. See DOI: 10.1039/c6ob01148a |
| ‡ These authors contributed equally to this work. |
| § The physicochemical properties were calculated based on scaffolds with all R groups counted as hydrogen atoms. |
| ¶ One major challenge for the production of this library lies in the apolar nature of the scaffold LS3, which resulted in lower success rates in the initial production attempts since standard purification conditions relying on preparative HPLC/MS proved to be unsuitable. After investigating various combinations of stationary and mobile phases, the best result was obtained by using a C18 column eluting with a gradient starting from 70% acetonitrile in aqueous ammonia (10 mM). |
| This journal is © The Royal Society of Chemistry 2016 |