Enantioselective N-heterocyclic carbene-catalyzed synthesis of saccharine-derived dihydropyridinones with cis-selectivity†
Abstract
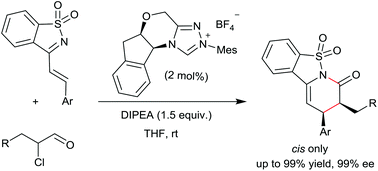
The enantioselective N-heterocyclic carbene-catalyzed [2 + 4] cyclocondensation of α-chloroaldehydes and saccharine-derived 1-azadienes was developed, giving the corresponding saccharine-derived dihydropyridinones in good yields with exclusive cis-selectivities and excellent enantioselectivities.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery

 Please wait while we load your content...
Please wait while we load your content...