Synthesis of cycloalkyl substituted purine nucleosides via a metal-free radical route†
Abstract
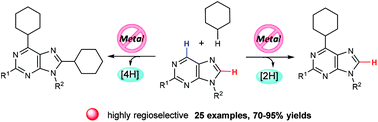
An efficient route to synthesize cycloalkyl substituted purine nucleosides was developed. This metal-free C–H activation was accomplished by a tBuOOtBu initiated radical reaction. By adjusting the amount of tBuOOtBu and reaction time, the selective synthesis of C6-monocycloalkyl or C6,C8-dicycloalkyl substituted purine nucleosides could be realized. Furthermore, uracil and related nucleosides were also suitable substrates, giving the C5-cyclohexyl substituted uracil derivatives in good yields with excellent regioselectivities.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery

 Please wait while we load your content...
Please wait while we load your content...