Chemical metrology
Analytical Methods Committee, AMCTB No. 76
First published on 14th November 2016
Abstract
The phrase chemical metrology generates a wide variety of responses from analytical chemists and a degree of confusion. This technical brief aims to dispel the mythology of metrology, explain important aspects and demonstrate the value of metrology to chemical measurements. It is shown that the basic principles of metrology have been applied since ancient times and served well for thousands of years as the key to consistent measurements. Application of the principles to physical measurements was formalised towards the end of the 19th century to meets the needs of industrialisation and global trade. The most recent change in global adoption of metrology took place at the end of the 20th century with the decision to extend the system to analytical chemistry. The brief describes how this decision is being implemented and the progress which has been made towards an international infrastructure for chemical and more recently biological measurements.
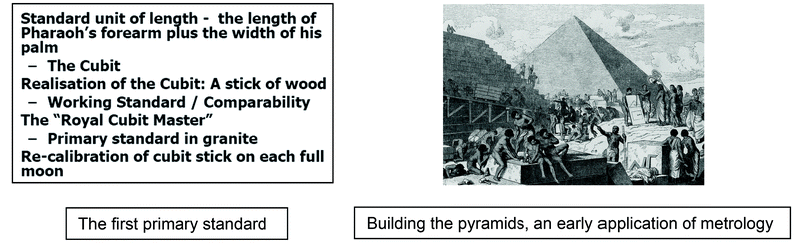
Measurements are as old as civilisation and the concepts of metrology have been applied for thousands of years. For example, building a pyramid was only possible if the many craftsmen employed in construction worked with the same unit of length. The Egyptians achieved this by defining a standard unit of length and establishing a practical means to disseminate it to the builders.
The Egyptian unit, the cubit, used the length of the Pharaoh's forearm to create a unique granite block as the master or primary standard of length. Distribution to the builders was based on calibrated wooden sticks, or secondary standards, which required regular re-calibration to ensure consistency over time. The so-called Great Pyramid has a base about 230 m in each direction, with a difference of only 58 mm among the four sides so the Egyptian system was very good indeed! This simple approach continues to the present day as the basis for achieving consistency of measurements, regardless of the unit. The Greek word metrology (literally the science of measurement) is now commonly associated with the implementation of systems of this type.
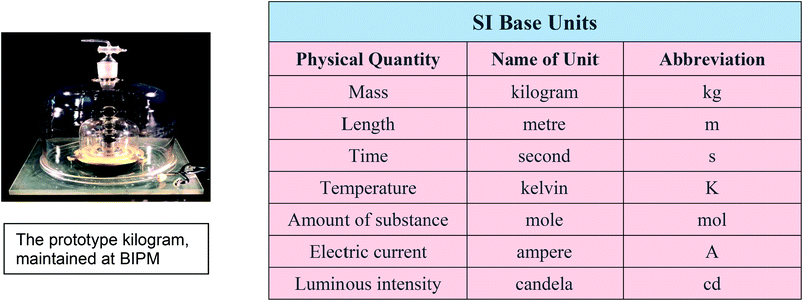
The process of linking everyday measurements to a unique primary standard or reference is known as establishing traceability. A result that can be linked to a primary standard, usually through a chain of calibrations, is metrologically traceable, or simply traceable, to that standard. Achieving this on a global scale requires a large and complex metrology system, which dates back to 1875 when an international treaty known as the Metre Convention was signed in Paris. Under the treaty, members of the Convention agreed to recognise a global primary measurement standard for each measurement quantity and to use metric units. A global infrastructure was established to develop and maintain the primary standards and disseminate traceable calibrations. This was centred on a new laboratory established at Sèvres near Paris, the Bureau International des Poids et Mesures (BIPM). This infrastructure has continued into the 21st century and comprises the BIPM, international committees to oversee and implement the underpinning measurement science, and collaborating National Measurement Institutes (NMIs) to disseminate traceable calibrations and standards locally.
The 1875 treaty envisaged that a unique artefact, the global primary standard, would be maintained at BIPM for each of the base measurement units but today this is true only for the prototype kilogram. It has become increasingly difficult to develop and maintain such standards to the level of accuracy required by today's science and technology. Since the introduction of the international system of units (the SI) in the 1960s, metrology has gradually moved to a system whereby each of the SI base units is defined in terms of fundamental scientific constants. This enables the primary artefact to be replaced by an experimental procedure which can be used to obtain the primary realisation of the unit.
Analytical chemists are familiar with the concept of traceability because the vast majority of chemical analysis methods require not only reliable chemical calibration standards but also accurate measurement of mass and/or volume. These physical quantities depend on calibrations that extend back to the primary realisations of the kilogram and metre respectively. The need for traceability of such calibrations has been accepted in analytical laboratories for many years and for accreditation to ISO 17025 it is mandatory to demonstrate appropriate traceability on all such calibration certificates. Traceability of chemical calibration standards is equally important but until relatively recent times implementation has been inhibited by the practical aspects. In particular, each specific chemical substance would need a separate reference point using the 19th century approach to metrology. This would have required the BIPM to maintain many thousands of global primary chemical standards, a task which is no longer necessary thanks to the introduction of the SI as mentioned above.
The base SI unit for chemistry (the mole) is defined in terms of a fundamental property, the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12. The definition of the mole also determines the value of the universal constant that relates the number of entities to amount of substance for any sample. This constant is called the Avogadro constant – although its value is not currently known beyond nine significant figures. As part of a wider re-definition of the SI base units, planned for 2018, it has been proposed that the mole be redefined in terms of a fixed and exact Avogadro constant and no longer on the basis of 0.012 kilogram of carbon 12. Any changes in uncertainty resulting from this will be invisible for all practical chemistry applications. It should be remembered that there is no “metrological requirement” to express the amount of a substance as a molar quantity. A numerical conversion is easily applied and, indeed, in most common applications the analyte is reported as mass or mass fraction rather than as a molar amount.
The direct approach to realising a molar amount of any substance is to weigh an amount of the pure substance corresponding to its atomic or molecular mass in grams. An “absolutely pure” sample of this substance is not needed provided its purity has been determined with an uncertainty that is consistent with the intended applications. Chemists have long prepared chemical calibration standards by weighing pure substances but accurately determining the purity of many important analytes is by no means a trivial task and can be the source of significant measurement errors. This was one reason for developing a metrological infrastructure to demonstrate their consistency and global traceability. Other reasons to establish such an infrastructure have also become apparent. The increase in global trade across national borders has made it increasingly important for different countries to show that their measurements agree with those of their trading partners. Issues such as climate change have brought about the recognition that environmental monitoring data need to be consistent on a global scale and over long periods of time.
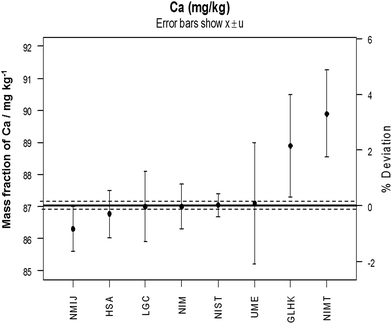
In 1993 the task of establishing a global infrastructure for chemical measurements was given to a new Consultative Committee for Metrology in Chemistry (CCQM) which first met in 1995. Given the scale of the task, the CCQM subsequently established working groups for each of the main chemical, and later biological, measurement areas. The main activity of the CCQM working groups is organising high level measurement comparisons between their members but they also collaborate on new measurement capabilities and assist new institutes to develop their capability. The comparisons are similar to a proficiency testing (PT) scheme but the aim is to achieve much smaller measurement uncertainty and closer agreement than would be normally be observed in PT schemes. This is essential as the objective of the CCQM participants is to deliver the topmost level of global traceability against which other laboratories can calibrate or validate their own measurements. Results from a typical CCQM comparison are shown in Fig. 1. Reports of all the comparisons organised by the CCQM are publicly available from a database maintained by the BIPM (http://kcdb.bipm.org/).
There is one important aspect in which the work of the CCQM differs from the vision of the 19th century metrologists who were concerned only with physical measurements. Chemical measurements often require determination of analytes in very complex matrices or at minute concentrations. This is achieved with complicated procedures involving steps such as extraction or separation of the analytes. Traceable calibration standards alone cannot ensure consistency of such measurements. This depends on validation of the methods, typically using certified reference materials (CRMs) with a matrix similar to the intended samples. Many applications of modern instrumental techniques, for example in metallurgy or geochemistry, also use matrix CRMs rather than pure chemical standards for calibration. Hence, the CCQM participants are working to demonstrate global consistency not only for pure substance calibration standards but also for matrix CRMs used for calibration and validation.
The work of CCQM has established a large global network of participating institutes to make the practical application of chemical and biological metrology a reality. This is achieved through the services offered by individual institutes, in particular the provision of a wide range of standards, reference materials and calibration services. The measurement methods used by the CCQM participants are not always greatly different from those of any laboratory making similar measurements. What does differ is the time and effort put into each application and into CCQM comparisons to ensure the consistency of results around the world over long periods of time. The aim of each national institute that participates in CCQM is to provide traceability of chemical or biological measurements at the highest level within its country or application area. The goal is demanding but working together on a global basis has stimulated progress and encouraged many more institutes to enter the field. This recent addition to the world of metrology is helping to ensure the future consistency of chemical and biological measurements around the world by using the same principles developed by the pyramid builders almost 5000 years ago.
Michael Sargent (LGC Limited)
This Technical Brief was prepared for the Analytical Methods Committee with contributions from members of the AMC Instrumental Analysis Subcommittee. It was approved by the AMC on 28/09/16.
| This journal is © The Royal Society of Chemistry 2016 |