Self-diffusion in nanocrystalline alloys
Abstract
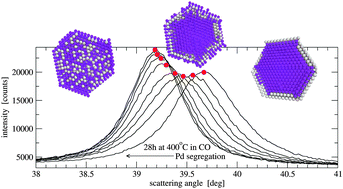
We report an operando XRD/MS experiment on a nanocrystalline Pd(70%)Ag(30%) alloy supported on silica (10 wt% of metal) monitoring slow reversible Pd (in CO) and subsequently Ag (in He) surface segregation at 673 K. XRD data following CO and He flow show structural changes that can be modeled and interpreted in terms of the diffusion phenomena within a typical metal nanocluster. Qualitative differences in the rate of both segregation processes suggest different diffusion mechanisms as the Pd segregation involves vacancy depletion. The experimental details suggest that this kind of experiment can provide a very sensitive response to subtle changes at the surface of nanoclusters. Segregation processes can be stopped at any time by lowering the temperature below 573 K which allows engineering of the metal surface e.g. preparing for a catalytic low temperature reaction on a well-defined surface.
- This article is part of the themed collection: Recent advances in the chemical physics of nanoalloys

 Please wait while we load your content...
Please wait while we load your content...