Anion-dependent spin crossover in solution for an iron(ii) complex of a 1H-pyrazolyl ligand†
Abstract
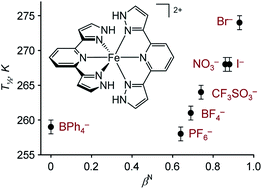
The spin-crossover equilibrium midpoint temperature (T1/2) in [Fe(3-bpp)2]X2 (3-bpp = 2,6-di{pyrazol-3-yl}pyridine) varies from 259 K when X− = BPh4− to 277 K when X− = Br−, at 10 mM concentrations in an acetone–water solvent mixture.
- This article is part of the themed collection: Supramolecular chemistry: self-assembly and molecular recognition

 Please wait while we load your content...
Please wait while we load your content...