What causes most errors in chemical analysis?
Analytical Methods Committee, AMCTB No 56
First published on 14th May 2013
Abstract
In the early 1980s, emerging evidence of poor inter-laboratory precision in routine analysis for trace contaminants led to major changes in the management of analytical chemistry. The adoption of the CODEX and later VAM principles encouraged laboratories to participate regularly in proficiency test (PT) schemes; to adopt validated analytical methods; to rely increasingly on certified reference materials for calibration and validation of their test methods; to implement effective internal QC; and (more recently) to obtain accreditation to standards such as ISO 17025.
As a result of these measures, and almost certainly with the benefit of continuing improvement in analytical equipment, PT scores in many sectors have shown significant and sometimes dramatic improvement over the past three decades. Yet PT schemes still sometimes show a high proportion of ‘poor’ scores, including a sprinkling of extreme values (as much as 4% according to one study). What causes such results? Textbooks provide lists of potential causes of error, but fail to identify those most commonly encountered in practice and most urgently in need of remedy. However, a recently published paper1 shows us a way forward.
Results from proficiency tests
A good understanding of the most common causes of error is essential if we intend to improve analytical results. An obvious place to look is in the results of proficiency testing schemes. Yet there are few published summaries of specific causes of poor PT scores following investigation by the scheme provider or within the laboratories concerned. There are good examples from clinical chemistry, but examples from general analytical chemistry are hard to find.There are many possible reasons for this: post-study investigation of individual cases is not the responsibility of the provider; data collection and its analysis are time-consuming; and the burden on participants of providing detailed information is high. Some of these barriers, however, can be overcome by means of a web-based study set up independently of the scheme's normal operation. A recent publication has provided a pilot example. The results, summarised here, suggest that learning to reduce basic human errors may be among the most effective ways of reducing the number of poor scores in PT and, by implication, generally.
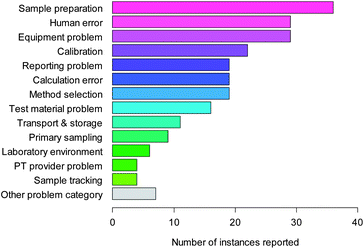 | ||
| Fig. 1 Causes of error in chemical analysis. | ||
The study in question was a voluntary-response survey in which PT participants in a number of different schemes were invited to give details of the causes of their most recent poor PT score. Most good laboratories have investigated a poor PT result at some time, so could provide useful information. Further, because electronic data collection could be limited to the specific causes, less information was needed per return than for a full survey of analytical methodology. The question of interest was, effectively, ‘What caused your last poor PT score?’
What caused your last poor PT score?
Fig. 1 shows the responses to this question. Sample preparation, equipment problems and human error are the top three categories in terms of response, with 41% of the responses within these three. Calibration errors, method selection, calculation error, reporting issues and test material problems form a natural intermediate group; the remaining responses (sample transport and storage, sample tracking, laboratory environment and the half-dozen other problems) form a low-response group including just under 20% of the total.The original paper indicated that within these broad categories, a few detailed causes of error stood out. Equipment failure was the most common single problem reported (8% of responses); dilution to volume in sample preparation the next (7%). Use of incorrect units in reporting, extraction problems, and transcription errors in data entry and reporting each accounted for about 5% of reported causes. ‘Human error’ was the second most important reported cause of poor PT scores.
This last category, however, did not include all of the features that could be attributed to human error. For example, ‘calculation error’ included errors such as incorrect formulae entered in spreadsheets—a human, rather than software, error. The report therefore identified all the detailed responses that could reasonably be regarded as examples of human error, and then counted the respondents who had recorded one or more of these responses. A total of 49 respondents (44% of the total) had reported one or more of these contributory causes. No other broad category was reported by so many respondents.
To err is human…
Simple human errors therefore represented the most common cause of poor PT scores in this survey. These findings are similar to the outcomes of often much larger studies in the clinical sector. Instrument problems, dilution errors, transcription errors in reporting and incorrect calibration are among the leading causes of poor results in clinical analysis too.This finding does not mean that laboratory managers can stop worrying about validation, accreditation, internal QC and traceability for their reference materials. Proper attention to these issues is a large part of the reason for the large number of acceptable results in PT. But the results do suggest that significant improvements could be made with more attention to the reduction of basic human errors.
…to forestall error is good practice
The implementation of quality management systems goes a long way to reducing error: what more can we do? AMC Technical Briefs No 49 lists some approaches that would catch a high proportion of simple mistakes.2 Simple transcription and reporting errors can be cut drastically by higher levels of automation. But perhaps the ‘bottom line’ is that we need to take another look at managing simple human error to achieve the next significant improvement in quality in chemical analysis.Afterthought
Laboratories that receive poor PT scores demonstrate, in addition to analytical problems, a lack of attention to their internal quality control systems. Routine IQC should have picked up any problems affecting the run of analysis before the result was reported. A correlation between good IQC and healthy PT scores was demonstrated some time ago.3
This Technical Brief was prepared for the Analytical Methods Committee by the Validation Subcommittee.
Further reading
- S. L. R. Ellison and W. A. Hardcastle, Accredit. Qual. Assur., 2012, 17, 453–464, DOI:10.1007/s00769-012-0894-2.
- AMC Technical Briefs No 49: “Sporadic Blunders”.
- M. Thompson and P. J. Lowthian, Analyst, 1993, 118, 1495–1500 RSC.
| This journal is © The Royal Society of Chemistry 2013 |


