Dendritic nanoconjugate containing optimum folic acid for targeted intracellular curcumin delivery†
Yunlan Fua,
Xuli Gaoa,
Ying Wanb,
Ju Zhangc,
Deling Kongc,
Zheng Wang*a and
Yanjun Zhao*a
aTianjin Key Laboratory for Modern Drug Delivery & High Efficiency, School of Pharmaceutical Science & Technology, Tianjin University, 92 Weijin Road, Tianjin 300072, China. E-mail: zhaoyj@tju.edu.cn; wangzheng2006@tju.edu.cn; Fax: +86-22-27404018; Tel: +86-22-27404018
bCollege of Life Science and Technology, Huazhong University of Science and Technology, Wuhan 430074, China
cInstitute of Biomedical Engineering, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin 300192, China
First published on 16th September 2014
Abstract
Based on surface-engineered poly(aminoamie) dendrimers, a novel type of targeted bifunctional nanoconjugate with optimum folic acid was designed to enhance the intracellular delivery of a highly pleiotropic active agent, curcumin.
Owing to the pleiotropic activities of curcumin, it has received increasing attention as a potential therapeutic agent against various diseases.1–3 However, due to its limited aqueous solubility (ca. 1 μg mL−1), chemical instability, rapid metabolism and systemic elimination, efficient delivery of curcumin has been challenging.4 Its degradation is rapid under neural (e.g. blood pH) and alklinic conditions, particularly at elevated temperature.5 Once absorbed, curcumin is also subjected to sulfation and glucuronidation at various tissue sites, resulting in the activity reduction.6 Thus, the poor bioavailability of curcumin is attributable to all of these factors. which makes it difficult to formulate an efficient aqueous curcumin delivery system.
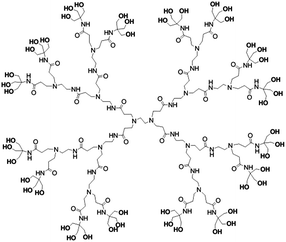
A diverse range of approaches have been employed to address the low bioavailability of curcumin, among which advanced nanotechnology has been the most promising one in offering a solution to substantially increase the pharmacological efficacy of curcumin in vivo.7 Dendrimers are unique polymeric nanocarriers with precise molecular uniformity, ultra-small size, and adjustable core–shell architecture.8,9 The multivalency of dendrimers is proportionally linked to their generation (G). For example, the number of the surface terminal groups on polyaminoamine (PAMAM) dendrimers increases exponentially with the dendrimer generation. The problem of generation-dependent cytotoxicity and haemocompatibility, and difficulty of mass production make high-generation dendrimers unsuitable in terms of their potential to be developed into pharmaceutical and biomedical applications.10 In contrast, low-generation dendrimers (G ≤ 3) are fairly easy to manufacture on a large-scale, but the quantity of terminal groups is inadequate, which limits the options during the process of surface engineering for desired dendrimers. We have modified the surface of a low-generation dendrimer, PAMAM, with tris(hydroxymethyl)aminomethane (Tris), which could triplicate the number of surface terminal groups of PAMAM without increasing its generation.11
The possibility of using PAMAM for curcumin loading has been extensively investigated in forms of physical encapsulation or chemical conjugation,12 although the efficiency of these dendrimers-based delivery systems is usually hampered by the absence of targeting moieties. It is well known that folic acid receptors are overexpressed in certain tumor cells, which may therefore be exploited to enhance tumor targeting of active agents. It was demonstrated that the optimized number of folic acid present at dendrimer surface was 5–7 due to the excellent balance of residence and dissociation between the carrier and cell target.13
The aim of this work was to improve the delivery efficiency of curcumin by a novel bifunctional dendrimer to which both curucmin and folic acid with defined ratio were conjugated.
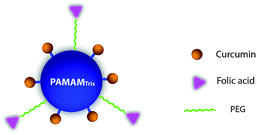
In this study, we took advantage of the PAMAMTris platform (Fig. 1), and generated a dendrimer–curcumin-co-folic acid nanoconjugate as targeted water-soluble nanocarrier in order to achieve enhanced curcumin delivery (Schemes 1 and 2). Briefly, curcumin and folic acid were co-conjugated to the exterior of PAMAMTris (G3). The additional introduction of hydrophilic poly(ethylene glycol) (PEG) to the PAMAMTris surface aimed to maintain its solubility and avoid aggregation; these desired features would extend the systemic circulation of the nanocarrier.
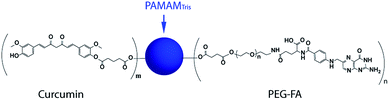 | ||
| Scheme 2 The chemical structure of bifunctional nanoconjugate: PAMAMTris–Cur-co-(PEG–FA). Cur, FA, and PEG are short for curcumin, folic acid, and poly(ethylene glycol), respectively. The structure of PAMAMTris was shown in Fig. 1. | ||
The synthesis details of the bifunctional nanoconjugate and related intermediate products are provided in ESI (Schemes S1–S3, ESI†). The mono-carboxyl-terminated curcumin (Cur-COOH) and carboxyl-terminated targeting moiety (FA–PEG-COOH) were sequentially conjugated to the surface of PAMAMTris via ester bonds (Fig. S2, ESI†). It could be seen from the 1H-NMR spectra that the number of conjugated Cur-COOH and FA–PEG-COOH was 9 and 6, respectively. Though it may result in precipitation of the nanoconjugate due to the lipophilic nature of curcumin, the density of conjugated curcumin on the dendrimer surface could be upregulated by increasing the feeding ratio between Cur-COOH and PAMAMTris.
The hydrodynamic diameter of PAMAMTris–Cur-co-(PEG–FA) was determined by dynamic light scattering at 37.5 ± 4.2 nm (Fig. S4†). Compared to the unmodified dendrimer whose hydrodynamic size was within 10 nm, the size expansion of nanoconjugate was due to the presence of surface modification groups, particularly PEG–FA. The result is consistent with the size obtained by transmission electron microscopy (Fig. S5†). The external surface of the generated nanoconjugate is negatively charged, as evidenced by the zeta potential of −13.1 ± 4.2 mV. This may be due to the presence of carboxyl group in folic acid. We therefore proposed that the nanoconjugate particles may be stabilized by electrostatic repulsion as well as the steric effect of PEG.
The drug loading of PAMAMTris–Cur-co-(PEG–FA) was 23.1 ± 1.5% (w/w), which is slightly higher compared to the loading value (ca. 18.5 ± 1.3%, w/w) of a recently reported linear polymer–drug conjugate micellar nanocarrier.14 In contrast to the previous micellar nanocarrier in which curcumin was loaded by physical encapsulation and chemical conjugation, curcumin loading in PAMAMTris–Cur-co-(PEG–FA) was achieved solely by covalent conjugation. This loading mode guarantees stability of the drug–dendrimer complexes, which is beneficial to minimize premature drug release. The manner of non-covalent association between the active compound and carriers often cause systemic toxicity and multiple side-effects.15
Drug release study was carried out in sink conditions with the aid of sodium dodecyl sulfate surfactant (5%, w/w) in pH 7.4 buffer at 37 °C (Fig. 2). Namely, the maximum drug concentration in the receiver fluid is less than 10% of the saturated drug solubility therein. Prior to 12 h, both PAMAMTris–Cur-co-(PEG–FA) and PAMAMTris–Cur exhibited rapid curcumin releasing profile before reaching plateau. At the end of 48 h, ca. 25% (w/w) of curcumin was detected in the release medium. A mass balance study revealed that curcumin recovery was only ca. 60% (w/w) at the end of the release experiments. This result is not surprising as curcumin is known unstable under alklinic and neutral conditions, particularly at higher temperatures.14 It was previously reported that ca. 90% of the naked curcumin degradated within 30 min when incubated in phosphate buffered saline (pH 7.2) at 37 °C.16 Likewise, we have also observed simultaneous curcumin release and degradation. Nevertheless, less degree of curcumin degradation is possible by lowering the surrounding temperature and/or pH, although these modified conditions lack physiologically relevance. As expected, biconjugate dendrimer exhibited comparable drug release rate when compared to the PAMAMTris–Cur control. This was because of the same mode of curcumin–dendrimer conjugate (i.e. ester bond). The low extent of drug release was simply a result of slow hydrolysis of ester bond that was the only way of curcumin release in current study. Regarding the current in vitro release experimental set-up, the receiver fluid contained no esterase that was believed able to speed up the breakdown of ester bond.9 Under acidic or base conditions, the ester bond could undergo higher degree of hydrolysis depending on the pH and temperature. However, under neutral circumstances (pH 7.4), the rate of hydrolysis is much lower. Our previous investigation conjugated curcumin to poly(lactic acid)-co-poly(ethylene glycol) via the ester bond; the result of drug release at pH 7.4 under sink conditions also revealed a similar release profile.14 The unique release profile of curcumin was a consequence of slow hydrolysis of ester bond and decent degree of curcumin degradation.
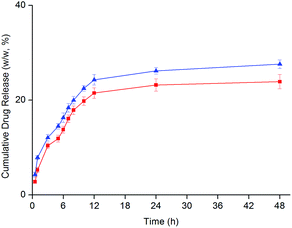 | ||
| Fig. 2 The cumulative curcumin release profiles from dendrimer nanoconjugates: PAMAMTris–Cur (triangle) and PAMAMTris–Cur-co-(PEG–FA) (square) (n = 3). | ||
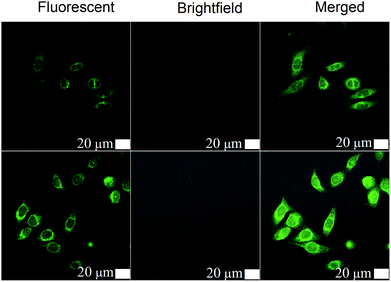
Using a human hepatocellular carcinoma cell line, HepG2, the intracellular uptake of curcumin was qualitatively analyzed by confocal laser scanning microscopy (CLSM) (Fig. 3). Since curcumin exhibits intrinsic green fluorescence, no additional immunofluorescent label was required.14 As expected, the bifunctional PAMAMTris–Cur-co-(PEG–FA) showed the strongest fluorescent intensity in comparison to the control group, dendrimer–drug conjugate without folic acid (i.e. PAMAMTris–Cur). Results of flow cytometry analysis are also consistent with the CLSM images (Fig. 4). The higher curcumin signal observed from the PAMAMTris–Cur-co-(PEG–FA) group may be a result of multivalent cooperative binding between folic acid (FA) and the FA receptors, leading to a longer residence time of the nanoconjugate at the cell surface, which in turn enhanced its cellular incorporation.17 A previous investigation demonstrated a dramatic enhancement of binding avidity (up to 5 orders of magnitude) between an FA-conjugated PAMAM and KB cells via multivalent effect.13 To increase the number of FA per dendrimer would increase the association rate constant while reduce the dissociation rate contant. The elevated number of FA also has a positive impact on binding affinity. However, this would generate additional barrier during cellular entry as the carriers would have difficulties to detach from the cell surface.
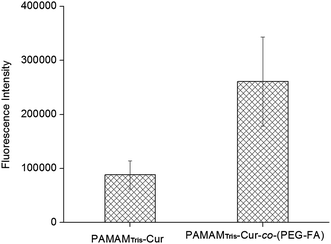 | ||
| Fig. 4 Flow cytometric analysis of mean curcumin fluorescence intensity from PAMAMTris–Cur and PAMAMTris–Cur-co-(PEG–FA). HepG2 cells were used and the excitation wavelength was set at 425 nm. | ||
It has been reported that nanocarriers with a moderate ligand concentration significantly improved cell binding in comparision to those with lower or higher levels of ligand densities.18 The mechanism of FA-mediated nanocarrier targeting is known as the result of prolonged residence at the plasma membrane rather than increased endocytosis. Hence, ligand density, receptor distribution, the type and length of linker molecules and the size of the nanocarrier, all of these factors are able to impact the degree of saturation of the binding effects, which in turn determine the targeting efficiency and consequently, therapeutical efficacy.19 In this study, we conjugated 6 FA ligands to the bifunctional nanoconjugate PAMAMTris–Cur, which resulted in substantial improvement of intracellular curcumin uptake. Indeed, the tumour targeting property of an anti-cancer dendritic nanocarrier containing ca. 5 FA molecules has already been reported both in in vitro and in vivo studies.13
In summary, the bifunctional PAMAMTris–Cur-co-(PEG–FA) nanoconjugate with defined ratio of drug/FA was generated to realize the targeted intracellular delivery of curcumin. Future work will focus on the cytotoxicity and in vivo performance of this system. Such nanoplatform is highly versatile as a selection of targeting ligands could be incorporated instead of FA used in this study. The hydradynamic size of the nanoconjugate can also be adjusted by manipulating the length of PEG to achieve passive targeting as a result of enhanced permeability and retention effect. We therefore conclude that the principle of multivalency with optimum targeting molecules can be widely exploited to load multiple active agents similar to curcumin for more diverse applications.
Acknowledgements
The work was supported by Tianjin Research Program of Application Foundation and Advanced Technology (13JCQNJC13300; 14JCZDJC38400) and National Natural Science Foundation of China (2134068; 81371705). Prof. Zhimou Yang from Nankai University and Dr Chen Li from Chinese Academy Of Medical Sciences & Peking Union Medical College kindly read through this manuscript and gave valuable suggestions.Notes and references
- A. Goel, A. B. Kunnumakkara and B. B. Aggarwal, Biochem. Pharmacol., 2008, 75, 787 CrossRef CAS PubMed.
- B. Joe, M. Vijaykumar and B. R. Lokesh, Crit. Rev. Food Sci. Nutr., 2004, 44, 97 CrossRef CAS.
- S. Saha, A. Adhikary, P. Bhattacharyya, T. Das and G. Sa, Anticancer Res., 2012, 32, 2567 Search PubMed; Y. Sadzuka, M. Nagamine, T. Toyooka, Y. Ibuki and T. Sonobe, Int. J. Pharm., 2012, 432, 42 CrossRef CAS PubMed; R. Misra and S. K. Sahoo, Mol. Pharmaceutics, 2011, 8, 852 CrossRef PubMed.
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, Mol. Pharmaceutics, 2007, 4, 807 CrossRef CAS PubMed.
- Y. J. Wang, M. H. Pan, A. L. Cheng, L. I. Lin, Y. S. Ho, C. Y. Hsieh and J. K. Lin, J. Pharm. Biomed. Anal., 1997, 15, 1867 CrossRef CAS PubMed.
- C. R. Ireson, D. J. L. Jones, S. Orr, M. W. H. Coughtrie, D. J. Boocock, M. L. Williams, P. B. Farmer, W. P. Steward and A. J. Gescher, Cancer Epidemiol., Biomarkers Prev., 2002, 11, 105 CAS.
- P. Sanphui, N. Goud, U. Khandavilli, S. Bhanoth and A. Nangia, Chem. Commun., 2011, 47, 5013 RSC; M. M. Yallapu, M. Jaggi and S. C. Chauhan, Curr. Pharm. Des., 2013, 19, 1994 Search PubMed.
- C. C. Lee, J. A. Mackay, J. M. J. Frechet and F. C. Szoka, Nat. Biotechnol., 2005, 23, 1517 CrossRef CAS; S. Svenson and D. A. Tomalia, Adv. Drug Delivery Rev., 2005, 57, 2106 CrossRef PubMed.
- A. R. Menjoge, R. M. Kannan and D. A. Tomalia, Drug Discovery Today, 2010, 15, 171 CrossRef CAS PubMed; Y. Shen, Z. Zhuo, M. Sui, J. Tang, P. Xu, E. A. Van Kirk, W. J. Murdoch, M. Fan and M. Radosz, Nanomedicine, 2010, 5, 1205 CrossRef PubMed; K. Kono, Polym. J., 2012, 44, 531 CrossRef.
- R. Duncan and L. Izzo, Adv. Drug Delivery Rev., 2005, 57, 2215 CrossRef CAS PubMed.
- Y. Zhao, L. Shen, Y. Wan, X. Zhu and Z. Wang, Colloids Surf., A, 2012, 403, 164 CrossRef CAS.
- W. Shi, S. Dolai, S. Rizk, A. Hussain, H. Tariq, S. Averick, W. L'Amoreaux, A. El Idrissi, P. Banerjee and K. Raja, Org. Lett., 2007, 9, 5461 CrossRef CAS PubMed; M. Mollazade, N. Zarghami, M. Nasiri, K. Nejati, M. Rahmati and M. Pourhasan, Clin. Biochem., 2011, 44, S217 CrossRef.
- S. Hong, P. R. Leroueil, I. J. Majoros, B. G. Orr, J. R. Baker and M. M. Banaszak Holl, Chem. Biol., 2007, 14, 107 CrossRef CAS PubMed.
- R. L. Yang, S. A. Zhang, D. L. Kong, X. L. Gao, Y. J. Zhao and Z. Wang, Pharm. Res., 2012, 29, 3512 CrossRef CAS PubMed.
- L. M. Kaminskas, V. M. Mcleod, C. J. H. Porter and B. J. Boyd, Mol. Pharmaceutics, 2012, 9, 355 CrossRef CAS PubMed.
- Y. J. Wang, M. H. Pan, A. L. Cheng, L. I. Lin, Y. S. Ho, C. Y. Hsieh and J. K. Lin, J. Pharm. Biomed. Anal., 1997, 15, 1867 CrossRef CAS PubMed.
- N. A. Licata and A. V. Tkachenko, Phys. Rev. Lett., 2008, 100, 158102 CrossRef PubMed.
- S. Sengupta and A. Kulkarni, ACS Nano, 2013, 7, 2878 CrossRef CAS PubMed.
- D. R. Elias, A. Poloukhtine, C. Popik and A. Tsourkas, Nanomedicine, 2013, 9, 194 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of nanoconjugate generation and characterization. See DOI: 10.1039/c4ra08315f |
| This journal is © The Royal Society of Chemistry 2014 |