A curing system of benzoxazine with amine: reactivity, reaction mechanism and material properties†
JiaQin Suna,
Wei Weia,
YaZhen Xua,
JieHao Qua,
XiangDong Liu*a and
Takeshi Endob
aKey Laboratory of Advanced Textile Materials and Manufacturing Technology, Ministry of Education, College of Materials and Textile, Zhejiang Sci-Tech University, Xiasha Higher Education Zone, Hangzhou 310018, P. R. China. E-mail: liuxd2007@gmail.com; Fax: +86-571-8684-3785; Tel: +86-571-8684-3785
bMolecular Engineering Institute, Kinki University, Kayanomori, Iizuka, 820-8555, Japan
First published on 28th January 2015
Abstract
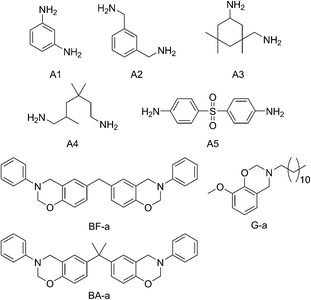
Five commercially available amines: m-phenylenediamine (A1), m-xylylenediamine (A2), isophorone diamine (A3), trimethylhexamethylenediamine (A4), and 4,4′-diaminodiphenyl sulfone (A5), were examined as nucleophilic hardeners for bis-benzoxazine monomers based on aniline paired with bisphenol-A (BA-a) or bisphenol-F (BF-a). The reactivities and reaction mechanisms of their mixtures with BA-a were investigated using FTIR and NMR spectroscopy, DSC, and HPLC techniques. It was found that BA-a rapidly cured with the amines upon heating at 120 °C or 150 °C. The cure rate was similar to the amine/epoxy curing process in practical uses, and significantly faster than the ring-opening polymerization of bulk BA-a. The possible reaction mechanism was supported by the experimental results and includes three successive steps: (i) nucleophilic substitution at the carbon atom (O–C–N) in the oxazine ring by the amine, (ii) thermal decomposition of the resulting aminomethanaminium structure, and (iii) electrophilic addition of the newly formed iminium ion with the aromatic ring to form stable aminomethylphenol structures. These findings are helpful to improve the thermosetting resins in terms of their chemical structure, material properties, and processability.
1. Introduction
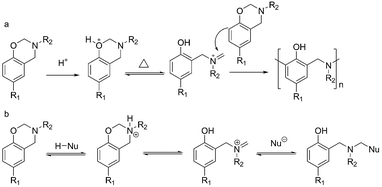
Since the discovery in 1994 that benzoxazines possess unique thermoset features that are better than conventional phenolic resins,1 this class of heterocyclic compounds has attracted great interest over the last 20 years.2–5 When compared with other polymers, polybenzoxazines exhibit significant advantages due to their mechanical and thermal properties, chemical and thermal stabilities, dimensional invariability during polymerization, and low surface energy.6 In particular, it is being gradually recognized that the most important and attractive characteristic of polybenzoxazines is the extraordinary flexibility in their molecular design.7 Benzoxazines are typically synthesized from phenol, amine, and formaldehyde via a Mannich reaction. Desired benzoxazines can be synthesized using specially selected phenol derivatives and primary amines. There have been a lot of successful reports on the development of benzoxazine monomers to improve material performance or expand the range of their applications,8 for example, lowering the polymerization temperature by introducing hydroxyl,9,10 acetylene,11,12 N-allyl,13 and carboxylic acid groups14 to benzoxazine compounds, increasing the cross-linking density by the incorporation of polymerizable groups,15–17 improving flame retardancy by phosphorylation of the cured products,18 and others.19–32To cure 1,3-benzoxazines, thermally accelerated polymerization has been the main focus for most researchers for a long time. Although the polymerization mechanism is complex and still poorly understood, the cationic polymerization mechanism shown in Scheme 1a has been widely recognized.19,20,33–35 The basic concept of the reaction mechanism is the protonation of the oxygen atom in the oxazine ring, followed by the formation of a zwitter iminium ionic structure, and its subsequent electrophilic substitution to the aromatic ring. Based on this point, several accelerators for the polymerization have been successfully developed.36–38
Besides this main methodology for curing benzoxazine monomers, another approach using ring-opening addition reactions, which is similar to curing epoxy with various hardeners, has recently attracted a lot of attention. The merits expected of this new strategy are the possibility to lower the curing temperature and the capability to further improve the materials performance by the careful selection of the cross-linking reagents. To date, very few compounds, including phenolic compounds,39–44 thiols,45–49 benzimidazoles,50 benzoxazoles,26,51,52 chitosan ammonium,53 and some polymers containing aromatic structures19,21,23 have been reported to construct chemical linkages with benzoxazines. A ring-opening addition reaction mechanism has been extended from the cationic polymerization mechanism mentioned above to explain the reactions (Scheme 1b). The hardeners donate a proton to the benzoxazine, resulting in an anion to attack the electrophilic iminium intermediate. Clearly, the benzoxazine acts as a nucleophile towards the proton in this case and the lone pair of the nitrogen in the oxazine ring is thought to be the most reactive position. On the other hand, it is not unreasonable to suppose that the benzoxazine acts as an electrophile due to the electron-deficient carbon atom found between the nitrogen and oxygen (O–C–N) and that the six-membered heterocycle has an irregular chair structure with considerable ring strain.54 Therefore, it is expected that a variation of reactants can open the oxazine ring via a nucleophilic attack to the electrophilic portion at the C8 carbon atom.
In this study, five commercially available amines: m-phenylenediamine (A1), m-xylylenediamine (A2), isophorone diamine (A3), trimethylhexamethylenediamine (A4), and 4,4′-diaminodiphenyl sulfone (A5), which are often used as industrial hardeners for epoxy resins, were selected as the nucleophilic reactants to harden benzoxazines (Scheme 2). Their reactivity with benzoxazine monomers was investigated using DSC and gel-point time analyses, and the reaction mechanism was studied using NMR and FTIR spectroscopy, HPLC and reaction activation energy (Ea) analyses. The general properties of the amine-cured polybenzoxazine resins, including their mechanical properties and thermal stability, were also evaluated.
2. Experimental section
2.1. Materials
The amines and benzoxazines used in the present study are listed in Scheme 2. m-Phenylenediamine (A1), m-xylylenediamine (A2), isophorone diamine (A3), trimethylhexamethylenediamine (A4), 4,4′-diaminodiphenyl sulfone (A5), 2,2-bis(4-hydroxyphenyl) propane (Bisphenol A), aniline, guaiacol, lauramine, paraformaldehyde, formaldehyde (37 wt% in water), 1,6-hexamethylenediamine (HMA), and benzylamine were purchased from Aladdin Reagent Co. (Shanghai, China). Bisphenol F based benzoxazine (BF-a, 75 wt% in butanone) was obtained from Huntsman investment Co., Ltd (Utah, USA). All other chemicals were of analytical grade and were purchased from Hangzhou Mike Chemical Agents Co. Ltd (Hangzhou, China).2.2. Measurements
1H-NMR spectra were recorded on an Avance AV-400 (400 MHz) NMR spectrometer (Bruker Co., Switzer-land) in CDCl3 or DMSO with TMS as an internal standard. FTIR spectra were obtained on a Nicolet 5700 FT-IR plus spectrometer (Nicolet Company, Madison, USA). UV-vis absorption spectra were measured on an UV-2550 spectrophotometer (Shimadzu Co., Japan). Viscosity was measured with a Brookfield CAP 2000+ viscometer, which offers a high shear rate with integrated temperature control for testing.An Agilent 1100 HPLC system (Agilent Technologies Co., Ltd, USA) with an Agilent ZORBAX-SBC18 (150 × 4.6 mm) column and a UV detector operating at 254 nm were used for all HPLC measurements. HPLC buffer A was 5 mM sodium phosphate aqueous solution mixed with phosphoric acid (NaH2PO4/Na2HPO4 = 1/1 in mol), and HPLC buffer B was pure acetonitrile. The eluting flow rate was 1 mL min−1. The linear gradient used was from 20% to 95% buffer B for 15 minutes.
DSC data were obtained from a DSC-1 differential scanning calorimeter (Mettler-Toledo corp., Switzer-land) at a heating rate of 10 °C min−1 in a nitrogen flow (35 mL min−1). TGA data were obtained from a TGA thermo-gravimetric analyzer (Mettler-Toledo corp., Switzer-land) at a heating rate of 20 °C min−1 under nitrogen atmosphere.
The mechanical tensile properties of the cured samples bound between aluminium sheets (bond surface area: 1.0 cm × 2.0 cm; binding thickness: fixed by glass beads of 0.2 mm diameter) were measured by an Instron-3367 instrument (Instron Co., Ltd, USA) (temperature, 25 °C; humidity, 30%; speed, 10 mm min−1). Dynamic mechanical analysis (DMA) was performed with a TA Instruments Q800 DMA (USA) with a heating rate of 5 °C min−1 from 25 to 250 °C at 1 Hz. The samples of 4 mm width were cut from films with approximate thickness of 0.4 mm.
2.3. Synthesis of 6,6′-(propane-2,2-diyl)bis-(3-phenyl-3,4-dihydro-2H-1,3-benzoxazine) (BA-a)
A mixture of aniline (18.80 g, 0.20 mol), paraformaldehyde (12.00 g), and bisphenol A (22.80 g, 0.10 mol) was stirred at room temperature for 15 min, heated at 100 °C for 30 min until the mixture became transparent. After the pressure was reduced to 0.01 MPa for 30 min to remove the water byproduct, the mixture was cooled to 25 °C, dissolved in ethyl ether (100 mL) and washed with NaOH solution (3.00 M, 3 × 100 mL) and water (3 × 100 mL). The organic phase was added with anhydrous MgSO4 (20.0 g), filtered, and dried under vacuum to obtain BA-a as a light yellow solid. Yield = 91%; mp 61 °C; 1H-NMR (CDCl3) 6.71–7.26, 5.33, 4.58, and 1.57 ppm; FTIR (KBr) 1328, 1230, 1157, 1030, and 946 cm−1.2.4. Synthesis of 3-dodecyl-8-methoxy-3,4-dihydro-2H-1,3-benzoxazine (G-a)
Laurylamine (4.63 g, 5.00 mmol) was first dissolved in anhydrous ethanol (10.00 mL). Formaldehyde solution (3.75 mL, 50.00 mmol) was slowly added and stirred for 20 min. Guaiacol (3.11 g, 25.00 mmol) was added to the mixture, stirred for 15 min and heated at reflux for 7 h. After recrystallizing from ethanol, and drying under vacuum, G-a was obtained as a white powder. Yield = 85%; mp 50 °C; 1H-NMR (CDCl3) 6.57–6.83, 4.96, 4.00, 3.87, 2.76, 1.25–1.57, and 0.88 ppm; FTIR (KBr) 1331, 1224, 1138, 1033, and 935 cm−1.2.5. General formulation of the benzoxazine/amine curing systems
BA-a (0.92 g, 2.00 mmol) and equivalent molar amount of amine were dissolved in chloroform (for A1–A4, 2.0 mL) or a mixed solvent of chloroform and ethanol (1/1, v/v, for A5, 4.0 mL), and dried under vacuum to obtain a uniform powder. These mixtures were employed as the samples for DSC, FTIR, TGA, and viscosity (redissolved in 3.0 mL of nitrobenzene) tests.G-a (0.15 g, 0.50 mmol) and an equivalent molar of benzylamine were dissolved in deuterated DMSO (6.0 mL), heated at 100 °C for a certain time, quenched with liquid nitrogen, and sampled for NMR analyses. These abovementioned sample mixtures (0.10 mL) were volumetric fed to 10 mL with methanol, and subjected to the HPLC measurements to detect unreacted benzylamine molecules.
The BF-a solution and an equivalent molar amount of the amine (A1–A5) were mixed (3 min) and degassed (3 min) with a conditioning mixer (AR-100, Thinky, Japan). Two pieces of filter paper (with an average pore size of 18 μm) were dipped in the BF-a/amine mixtures, clamped using polyimide sheets, dried under vacuum to remove the solvent, and cured at 120, 150, and 180 °C for 2 h. The resulted composite samples were subjected to DMA tests.
3. Result and discussion
3.1. Reactivity of amines toward benzoxazine
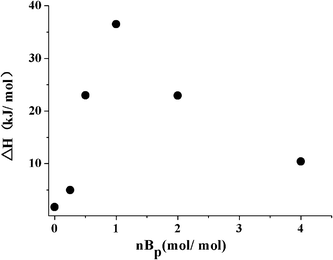
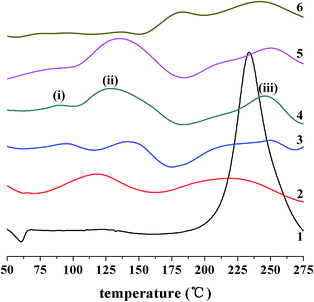
BA-a/A1 mixtures in a variety of molar ratios were tested using DSC at a heating rate of 10 °C min−1 under a nitrogen atmosphere. The incorporation of A1 leads to considerable exothermic peaks (Fig. SI 1†) at low temperature ranging from 100 to 200 °C. Because the ring-opening polymerization of neat BA-a takes place at high temperature (>200 °C), these peaks suggest that BA-a reacts with the amine in different ways. To estimate the stoichiometry ratio of the reactants, we defined BA-a moles per one mol amine in the reactive mixtures as nBp to describe BA-a dosage, and calculated the enthalpy values of the peaks formed at <200 °C as ΔH. The relationship between the ΔH and nBp is displayed in Fig. 1. The maximum ΔH value was obtained when nBp was 1.0, which means that one amine group approximately reacts with one oxazine ring.Based on the roughly stoichiometric estimate, we sequentially investigated the curing reactions of five amines (Scheme 2) with equimolar BA-a, using a dynamic DSC process from 50 to 300 °C. The DSC profiles are shown in Fig. 2 and the important data selected from the DSC tests summarized in Table 1. In contrast with the test of neat BA-a without amine, the addition of alkylamines A2, A3, and A4 results in two distinctively exothermic peaks before the last peak occurs. However, with arylamines A1 and A5, only one peak was distinguishable to the control test. The next notable fact was that the peak temperatures of the alkylamine mixtures were very low (centered at ∼75 °C), suggesting that the reaction between BA-a and the amines tested is active and rapid. In addition, calculation of the enthalpy values indicates that the amines are responsible for reduction in the reaction enthalpy values by a third. Therefore, several interesting deductions can be summarized from these DSC results: (1) there are at least two reaction mechanisms that dominate the curing process occurring at different stages; (2) the reactivity of the amines is determined by their chemical structures, i.e., alkylamines are generally more reactive than arylamines; and (3) the reaction of BA-a with amine is slightly exothermic, in contrast to the large enthalpy value of the thermally induced ring-opening polymerization of bulk BA-a.
| Curing systemb | Peak 1 | Peak 2 | Peak 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TO (°C) | Tp (°C) | ΔH (J g−1) | TO (°C) | Tp (°C) | ΔH (J g−1) | TO (°C) | Tp (°C) | ΔH (J g−1) | |
a DSC data were obtained at a heating rate of 10 °C min−1 in nitrogen atmosphere.b BA-a/amines are in molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. |
|||||||||
| Pure BA-a | — | — | — | — | — | — | 213 | 234 | 309 |
| BA-a/A1 | — | — | — | 79 | 122 | 42 | 158 | 224 | 70 |
| BA-a/A2 | 68 | 94 | 5 | 112 | 145 | 17 | 178 | 220 | 27 |
| BA-a/A3 | 65 | 95 | 4 | 103 | 133 | 50 | 185 | 247 | 45 |
| BA-a/A4 | 57 | 80 | 10 | 95 | 134 | 63 | 185 | 244 | 37 |
| BA-a/A5 | — | — | — | 157 | 184 | 17 | 206 | 254 | 46 |
To compare the reactivity of the five amines, we further explored the increase in viscosity behaviour of the reactive BA-a/amine (1/1, mol mol−1) mixtures using a Brookfield viscometer. The mixtures were isothermally cured at 120 °C and 150 °C, and their gel time points defined as the moment that the viscosity suddenly increases. The viscosity of a thermosetting system generally corresponds to the curing reaction rate and is often used as an index to analyse the kinetics of polymerization. As shown in Fig. 3 and Table SI 1,† the five amines significantly accelerate the curing reaction rate. In contrast with bulk BA-a, which shows little change in viscosity at the heating temperatures tested, the BA-a/amine mixtures display a rapid increase in viscosity in times ranging from minutes to hours. A comparison of A1 and A5 shows an obvious difference, which was attributed to the presence of the electron-withdrawing sulfone group in A5, decreasing the amine's reactivity towards BA-a.
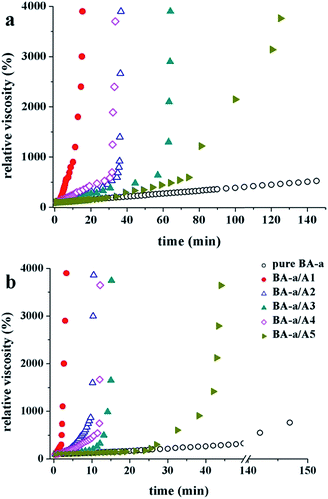 | ||
| Fig. 3 The time dependence of viscosity during the isothermal curing of the reactive mixtures at (a) 120 °C and (b) 150 °C. | ||
3.2. Mechanical properties and thermal stability of the amine cured benzoxazine resins
Based on the preliminary understandings gained on the reactivity of the benzoxazine/amine mixtures, we then paid attention to the mechanical and thermal properties of the thermosetting resins.First, a series of the BA-a/amine cured resins binding two aluminum sheets were investigated under an initial strain rate of 10 mm min−1. Table 2 summarizes the curing conditions, the engineering stress, and the stretch ratio for each mixture tested. The comparison between the control tests and bulk BA-a cured via the different process revealed that the ring-opening polymerization of BA-a needs high temperatures and long curing time. In contrast, the first three runs show that the addition of amine could simplify the curing process, in both the curing temperature and curing time. For example, the breaking strength of the second sample (run 2) that was cured within one hour is very close to that of the third sample (run 3), which can be recognized as a completely cured product. Moreover, the basicity of the amine seems to be an important factor affecting the mechanical properties. This was reflected from the strength data obtained from the alkylamine samples (runs 5–7), which are significantly stronger than that of the arylamine samples (runs 4 and 8). It is noteworthy that the five amines can enhance the mechanical strength of the cured BA-a resins, when compared with the control tests.
| Runs | Amine/benzoxazine | Curing process | Breaking strength (MPa) | Strain (%) |
|---|---|---|---|---|
| a The mean ± SD, the average values were obtained from 5 tests. | ||||
| Control 1 | —/BA-a | 120 °C/2 h, 150 °C/2 h | 18.53 ± 3.93a | 3.26 ± 0.11 |
| Control 2 | —/BA-a | 120 °C/2 h, 150 °C/2 h, 180 °C/2 h | 53.01 ± 13.68 | 4.96 ± 0.72 |
| 1 | A4/BF-a | 120 °C/1 h | 43.37 ± 4.90 | 4.48 ± 0.32 |
| 2 | A4/BF-a | 120 °C/0.5 h, 150 °C/0.5 h | 139.16 ± 21.06 | 5.63 ± 0.99 |
| 3 | A4/BF-a | 120 °C/2 h, 150 °C/2 h, 180 °C/2 h | 152.18 ± 18.75 | 5.96 ± 0.43 |
| 4 | A1/BA-a | 120 °C/2 h, 150 °C/2 h | 48.29 ± 10.97 | 4.42 ± 0.28 |
| 5 | A2/BA-a | 120 °C/2 h, 150 °C/2 h | 64.49 ± 16.3 | 5.84 ± 0.95 |
| 6 | A3/BA-a | 120 °C/2 h, 150 °C/2 h | 128.57 ± 37.33 | 8.51 ± 2.51 |
| 7 | A4/BA-a | 120 °C/2 h, 150 °C/2 h | 141.33 ± 28.79 | 7.27 ± 1.10 |
| 8 | A5/BA-a | 120 °C/2 h, 150 °C/2 h | 55.38 ± 9.19 | 3.26 ± 0.11 |
Next, DMA was used for the evaluation of the crosslinked polymers prepared from the heated BF-a/amine mixtures. As shown in Fig. 4a, the DMA curves indicate that the amine cured resins exhibit high storage modulus (ranged 3.5–6.5 GPa), which can be attributed to the high cross-linking of the curing system (Table SI 3†). Compared to bulk BF-a, the amine cured resins except from amine A5 achieve approximately same storage modulus, and the amine A2 results in a higher value of 6.5 GPa at 25 °C. The tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curves (Fig. 4b) of the amine cured resins show the viscoelastic transition of the crosslinked polymers. Their glass transition temperatures (Tg) as defined by the tan
δ curves (Fig. 4b) of the amine cured resins show the viscoelastic transition of the crosslinked polymers. Their glass transition temperatures (Tg) as defined by the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peak present interesting trends for the five amines. The arylamine samples (A1 and A5) have high Tg values (165–180 °C) similar to that of bulk BF-a, but the alkylamine cured resins show lower Tg values lesser than 105 °C. This fact is easy to understand as the rigid benzene rings limits the movement and rearrangement of the polymer chain segments.
δ peak present interesting trends for the five amines. The arylamine samples (A1 and A5) have high Tg values (165–180 °C) similar to that of bulk BF-a, but the alkylamine cured resins show lower Tg values lesser than 105 °C. This fact is easy to understand as the rigid benzene rings limits the movement and rearrangement of the polymer chain segments.
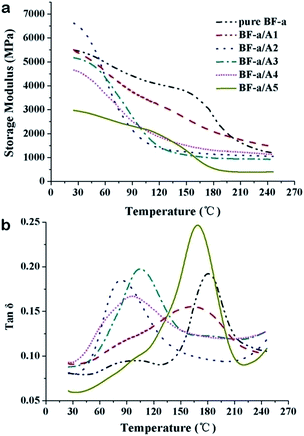 | ||
Fig. 4 DMA spectra of the amine-cured resins (cured at 120 °C/2 h, 150 °C/2 h, 180 °C/2 h). (a) Storage modulus; (b) tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ. δ. | ||
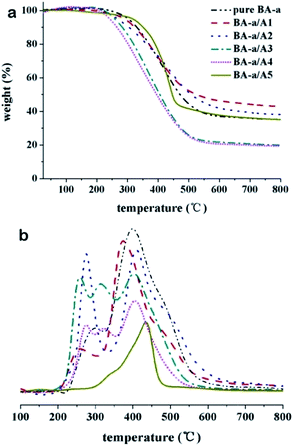
Furthermore, the thermal stability of the cured thermosetting resins was investigated using TGA under nitrogen atmosphere. Fig. 5 and Table SI 2† show the TGA thermograms and data collected from these thermograms, respectively. The TGA profiles indicate that amines having aromatic structures (A1, A2, and A5) can increase the char yield at 800 °C. For instance, the BA-a/A1 resin reaches up to 43%, which is clearly higher than that of neat polyBA-a (35%). On the contrary, alkylamines (e.g., A3 and A4) decrease the char yield to ∼20%. This result may be attributed to the increased quantity from the aromatic rings.
Theoretically, aliphatic chains are easier to decompose into gaseous molecules such as ethylene when compared to aromatic rings. Interestingly, the addition of A5 results in improved thermal stability when compared to the neat polyBA-a resin. The main degradation of the A5-cured resin occurs at ∼440 °C, while neat polyBA-a resin degrades at ∼400 °C. However, in the case of alkylamines (A2–A4), the degradation temperatures of the amine-cured resins are lower than that found with neat polyBA-a, implying that the aliphatic chains negatively affect the thermal stability of the thermoset resins.
3.3. Experimental observations associated with the reaction mechanism between the amine and benzoxazine
The abovementioned experimental data demonstrate that the amines not only accelerate the benzoxazine curing process but also improve the mechanical or thermal properties of the cured products. These results stimulated us to further study the possible reaction mechanism between benzoxazine and amine.Dynamic DSC scans were performed at four constant heating rates to investigate the five BA-a/amine curing systems. Table 3 summarizes their peak temperatures and their activation energy (Eap) values calculated to characterize their reactivity. According to Kissinger's method,55,56 the relationship between DSC peak temperature Tp in K and the heating rate β is of the following form:
| ln(β/Tp2) = ln(AR/Eap − Eap)/RTp |
As shown in Table 3, the five benzoxazine/amine curing systems show a lower Eap value than that for the polymerization of pure BA-a. Their Eap values follow an order of: arylamine > alicyclic amine > alkylamine, which is in good agreement with their basicity.
Then, we monitored the chemical changes of the BA-a/amine curing system with equivalent amounts using FTIR spectroscopy. 1,6-Hexamethylenediamine (HMA) was used as a replacement for amine A4 to simplify the IR spectrum, and the curing temperature was fixed at 100 °C to slow down the reaction rate. As shown in Fig. 6, the spectrum of the initial mixture without heating shows three characteristic peaks attributed to BA-a: the peak at 1230 cm−1 corresponds to the anti-symmetric stretching of the C–O–C bond in the oxazine ring, and the peaks at 1497 and 946 cm−1 are assigned to the trisubstituted benzene ring. In addition, the shoulder peak at 1580 cm−1 was assigned to the primary amine group of HMA. With the passage of cure time, the characteristic peaks of the reactants gradually decreased or shifted in the subsequent spectra. For example, three new and important peaks, corresponding to new covalent bonds, were observed at 1180, 1480, and 3407 cm−1; they were attributed to C–N–H bond stretching of a secondary amine, the tetra-substituted aromatic ring and phenolic OH group, respectively. The FTIR spectroscopic evidence suggests that the reaction between the amine and benzoxazine follows a ring-opening addition reaction mechanism.
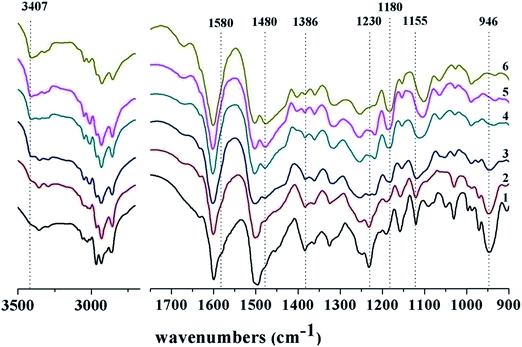 | ||
| Fig. 6 FTIR spectra of the reactive BA-a/HMA mixture (1) heated at 100 °C for (2) 10, (3) 20, (4) 30, (5) 60, and (6) 90 min. | ||
The monofunctional benzoxazine, 3-dodecyl-8-methoxy-3,4-dihydro-2H-1,3-benzoxazine (G-a), was synthesized from guaiacol, laurylamine, and formaldehyde as described in our earlier reports.57,58 The ortho-position on the phenolic ring is blocked and therefore complex polymerization can be avoided. The mixtures of G-a and benzylamine with an equivalent molar ratio were dissolved in deuterated DMSO and subjected to NMR spectroscopic analyses after heating for different times. As shown in Fig. 7, the 1H NMR spectra of the mixtures reveal obvious signal changes in the methylene proton region (δ = 5.0–3.5 ppm). For benzoxazine, the resonances at δ = 3.89 and 4.80 ppm were assigned to the –Ph–CH2–N– (i′) and –OCH2–N– (ii′) protons of the oxazine ring, respectively. In addition, the characteristic peak at δ = 3.71 ppm is responsible to the methoxy group. The other reactant, benzylamine, gives a signal at δ = 3.74 ppm (iii′) corresponding to its methylene protons. Because the methoxy group attached to the benzene ring is chemically stable at a mild heating temperature of 100 °C, the peak at δ = 3.71 ppm can be used as a reference for the other chemicals during the entire reaction process. After heating the mixture for a short time, a number of new signals appeared in the region 4.0–3.5 ppm (i, ii, and iii), suggesting the formation of new methylene groups. These peaks reasonably support the scheme shown in Fig. 7: (1) they increased with reaction time; (2) the integrated areas are consistent with their parent compounds (e.g., the sum of the pair peaks of i and i′ is nearly constant); (3) the assignment of the peaks to the methylene protons is logical. In addition, three peaks appeared in the range from δ = 3.5–3.3 ppm upon heating, which remained small and almost unchanged during the entire reaction process. This phenomenon implies that these small peaks are caused by the by-products formed via side reactions, especially those involving the secondary amine formed during the ring-opening addition reaction. However, although a long heating process was examined, the characteristic peaks of the oxazine ring (at δ = 3.89 and 4.80 ppm) were still observed in the NMR spectrum, which means that a considerable amount of G-a was unreacted.
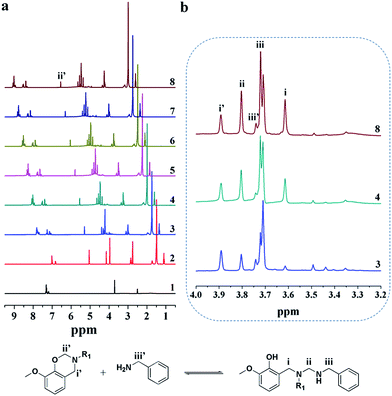 | ||
| Fig. 7 NMR spectra of benzylamine (1), G-a (2) and the reactive mixture, G-a/benzylamine heated at 100 °C for (3) 0.5, (4) 10, (5) 20, (6) 30, (7) 60, and (8) 90 min. | ||
To examine the conversion of the reactants during the heating process, we used HPLC equipped with a C18 reversed-phase column to separate and measure the benzylamine quantity in the mixtures heated for different times, and the results are shown in Fig. 8. The reaction rate of benzylamine is very rapid in the early stages, but gradually slows down to zero as the reaction progresses. This kinetic relationship shows that the ring-opening addition reaction between benzoxazine and the amine is reversible. In fact, this deduction has surprised us because a reversible reaction with kinetic features like those found in Fig. 8 (about 40% benzylamine residue) cannot make any cured thermosetting resins.
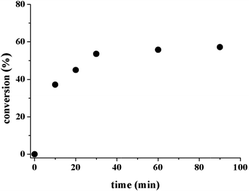 | ||
| Fig. 8 The conversion of benzylamine in the reactive mixture, G-a/benzylamine heated at 100 °C (benzylamine was detected by a HPLC method). | ||
The doubt whether or not free reactants exist in the cured resins leads us to carry out further investigations. We compared the FTIR spectra of two types of the cured BA-a/amine resins (Fig. SI 2 and 3†). One was heated at a higher temperature to complete the curing reaction, and the other was heated at 100 °C for 1.0 h to simulate the reaction that occurred in the NMR samples. As a result, the characteristic peaks attributed to the primary amine group were found only in the spectra of the products cured by arylamine (A1 and A5). We next extracted the completely cured resins (BA-a cured by the five commercial amines at 120 and 150 °C for 2 h, successively) with methanol in a Soxhlet extractor and analysed the extracting solution using a sensitive GC-MS method (with a minimum detectable quantity of <150 ng mL−1), and no amines were detected. However, when the amine was changed to 2,4,6-trimethylaniline (TMA) and 2,4,6-trichloroaniline (TCA), 27% and 35% residues of the amine were detected, respectively (Fig. SI 4†).
When the reactive mixtures of G-a/benzylamine were heated in their deuterated DMSO solution at higher temperatures, as shown in Fig. 9, more peaks appeared in the NMR spectra, indicating that a lot of additional methylene groups were formed. Based on the previous report,32 the NMR peaks in the range of δ = 4.9–4.7 ppm were attributed to various Ar–OCH2– structures, and peaks in the range of δ = 3.9–3.4 ppm were assigned to a wide variety of Ar–CH2–N–, Ar–CH2–Ar–, and N–CH2–N– structures. In short, these results suggest that following the reversible reaction between benzoxazine and the amine, complex reaction mechanisms will dominate the curing process at elevated temperatures.
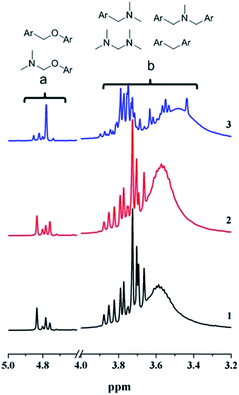 | ||
| Fig. 9 NMR spectra of the reactive mixture, G-a/benzylamine heated at 150 °C for (1) 0.5 h and (2) 1 h, and (3) heated at 180 °C for 2 h. | ||
3.4. The proposed reaction mechanism
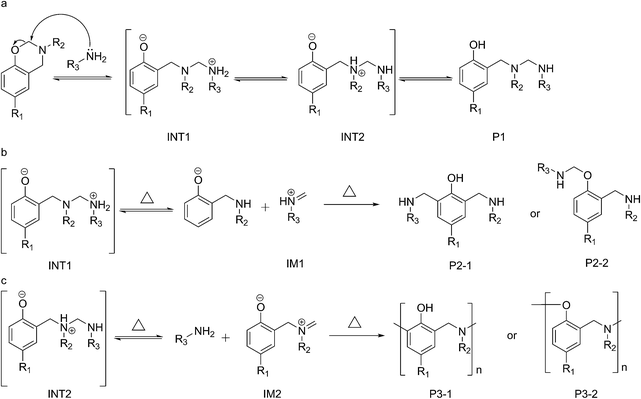
The cationic ring-opening polymerization mechanism proposed in Scheme 1a has been widely accepted by most researchers. A recent report has demonstrated a competitive pathway through phenoxy structures that finally convert to the phenolic polymers upon heating up to 160 °C.35 Furthermore, some special compounds, such as phenolic compounds, thiols, and chitosan ammonium, were used to react with benzoxazines42,46,49,53,59,60 and a new mechanism, described as a ring-opening addition reaction mechanism (Scheme 1b), has been proposed.46 However, these mechanisms are not applicable for the abovementioned experimental results. For example, most amines have a pKa > 30 (in comparison to thiols with a pKa 10–11 and ammonium ion pKa 4–11), meaning that fewer protons can separate to form the anionic conjugate base (RNH2−). Another contradiction was reflected when comparing the reactivity of BA-a towards the five amines: an electron-withdrawing group like sulfone should theoretically stabilize the anionic conjugate base (RNH2−) and increase its reactivity towards BA-a, but we obtained the opposite finding in our experimental results.Herein, we propose a detailed mechanism outlined in Scheme 3, in which three possible pathways dominate the curing process for the benzoxazine/amine mixtures at different stages. In the first reaction, the methylene group (at C8) between the nitrogen and oxygen is the most vulnerable position for the nucleophilic attack of the amine both in terms of its partial charge and ring strain. As shown in Scheme 3a, a typical nucleophilic substitution reaction results in a zwitter ionic intermediate (INT1), which further produces another intermediate (INT2) and the product compound (P1) by proton transfer. In theory, the reactivity of the amine for this reaction is determined by its basicity. This conclusion is in good agreement with the small DSC peaks (at <120 °C) shown in Fig. 2, which were only observed with the alkylamine mixtures (in contrast with the arylamines, in which the DSC peaks were very small or undetectable).
Furthermore, the NMR data (Fig. 7) and the HPLC results (Fig. 8) further demonstrate that the first reaction is reversible. The two intermediates are thermally unstable and decompose upon heating at an elevated temperature (Scheme 3b and c), releasing the iminium ions IM1 and IM2 that are very reactive with aromatic rings and phenolic hydroxyl groups, and so should go on and produce P2 and P3 products through electrophilic substitution reactions (Scheme 3b and c). The two intermediates, INT1 and INT2, are a competitive pair in the first reaction. Their competitive advantage, which depends on which quaternary amine is favourable, determines the dominant pathway in the curing process and the dominant structures (P2 or P3) in the cured resin. In the cures of BA-a, P2 should be the main structure in the cured resin because the quaternary alkylamines (pKa 10–11.5) are more stable than that of the aromatic amines (pKa 4–6). In the DSC profiles of the alkylamine mixtures, the second reaction should be responsible for the peaks at 120–170 °C, as the order of the Eap values calculated from the peaks agree with the basicity for the amines.
On the other hand, when the aromatic amines (A1 and A5) are used to cure BA-a, the intermediate of INT2 will take little advantage over INT1. These results in the formation of the P3 structures, and a few free amino groups will remain in the cured resin. This inference is supported by the FTIR spectra (Fig. SI 2 and SI 3†) obtained from the resin samples cured at 100 °C or at 120 and 150 °C for 2 h, as the primary amine groups are detected only from the arylamine (A1 and A5) cured resins. Because aromatic protons in the aromatic amines also react with the iminium ions (IM1 and IM2), aromatic amines can be cross-linked without the amino reactions. When benzoxazine is cured with 2,4,6-tri-substituted amines (TMA and TCA), more amine residues are detected in the cured resins (Fig. SI 4†). Since the residues of TCA are more than that of TMA, the results shown in Fig. SI 4† also support the competitive relevance between Scheme 3b and c. The interpretation is also applicable to the DMA test of amine A5, which largely decreases the storage modulus and the crosslinking density than bulk BF-a (Table SI 3†).
With the abovementioned mechanism in mind, not only the resin's structure, which is used to synthesise the benzoxazine monomer but also the curing reaction rate, which is used to cure the benzoxazine through a ring-opening addition reaction, can be improved by choosing appropriate amine pairs.
4. Conclusions
In summary, we have demonstrated the excellent nature of the ring-opening addition reaction of benzoxazine with an amine for the preparation of high performance thermosetting polymers. In contrast with the ring-opening polymerization of bulk BA-a, the addition of an amine not only achieved a much faster polymerization at mild temperatures (such as 120 °C or 150 °C), but also directly affected the characteristics of the amine thermally-cured benzoxazine resin. This fact was experimentally verified using five commercially available amines: m-phenylenediamine, m-xylylenediamine, isophorone diamine, trimethylhexamethylenediamine, and 4,4′-diaminodiphenyl sulfone as the hardeners of BA-a monomer. The Eap values calculated from the dynamic DSC scans of the BA-a/amine curing system revealed an expectable order of: arylamine > alicyclic amine > alkylamine, which is in good agreement with the basicity of the amines tested. The analysis of the FTIR and NMR spectra, and HPLC results further suggest that the curing reaction mechanism between the amine and benzoxazine involves: (1) a reversible reaction, involving the nucleophilic attack of the amine towards the benzoxazine to give zwitter ionic products with both phenolate and aminomethanaminium structures; (2) by heating at elevated temperature, the aminomethanaminium moiety decomposes to an iminium ion, and proceeds to take part in an electrophilic substitution reaction with the aromatic ring to form a stable aminomethylphenol structure. By addition of a suitable amine to the benzoxazine monomer, flexibility in the molecular design could be achieved to give the desired structures, and the processability could be improved in terms of shorter curing time and lower curing temperature.Acknowledgements
This study was financially supported by the Natural Science Foundation of Zhejiang Province (LY12E03007), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, the State Education Ministry (1101603 C).Notes and references
- X. Ning and H. Ishida, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 1121 CrossRef CAS.
- N. N. Ghosh, B. Kiskan and Y. Yagci, Prog. Polym. Sci., 2007, 32, 1344 CrossRef CAS PubMed.
- B. Kiskan, N. N. Ghosh and Y. Yagci, Polym. Int., 2011, 60, 167 CrossRef CAS.
- J. C. Ronda, G. Lligadas, M. Galia and V. Cadiz, React. Funct. Polym., 2013, 73, 381 CrossRef CAS PubMed.
- T. Endo and A. Sudo, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 4847 CrossRef CAS.
- Y. Yagci, B. Kiskan and N. N. Ghosh, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 5565 CrossRef CAS.
- A. D. Baranek, L. L. Kendrick, J. Narayanan, G. E. Tyson, S. Wand and D. L. Patton, Polym. Chem., 2012, 3, 2892 RSC.
- K. D. Demir, B. Kiskan, B. Aydogan and Y. Yagci, React. Funct. Polym., 2013, 73, 346 CrossRef CAS PubMed.
- B. Kiskan, B. Koz and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 6955 CrossRef CAS.
- R. Kudoh, A. Sudo and T. Endo, Macromolecules, 2010, 43, 1185 CrossRef CAS.
- A. Chernykh, T. Agag and H. Ishida, Macromolecules, 2009, 42, 5121 CrossRef CAS.
- K. D. Demir, B. Kiskan and Y. Yagci, Macromolecules, 2011, 44, 1801 CrossRef.
- H. Oie, A. Sudo and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 5357 CrossRef CAS.
- S. F. Li and T. Zou, J. Appl. Polym. Sci., 2012, 123, 922 CrossRef CAS.
- Y. Cheng, L. Kong, Z. Ren and T. Qi, High Perform. Polym., 2013, 25, 980 CrossRef PubMed.
- Y. Cheng, J. Yang, Y. Jin, D. Deng and F. Xiao, Macromolecules, 2012, 45, 4085 CrossRef CAS.
- Y. Cheng, T. Qi, Y. Jin, D. Deng and F. Xiao, Polymer, 2013, 54, 143 CrossRef CAS PubMed.
- H. C. Chang, H. T. Lin and C. H. Lin, Polym. Chem., 2012, 3, 970 RSC.
- I. Hamerton, L. T. McNamara, B. J. Howlin, P. A. Smith, P. Cross and S. Ward, Macromolecules, 2014, 47, 1935 CrossRef CAS.
- I. Hamerton, L. T. McNamara, B. J. Howlin, P. A. Smith, P. Cross and S. Ward, Macromolecules, 2013, 46, 5117 CrossRef CAS PubMed.
- I. Hamerton, L. T. McNamara, B. J. Howlin, P. A. Smith, P. Cross and S. Ward, Macromolecules, 2014, 47, 1946 CrossRef CAS.
- B. Hanbeyoglu, B. Kiskan and Y. Yagci, Macromolecules, 2013, 46, 8434 CrossRef CAS.
- O. S. Taskin, B. Kiskan and Y. Yagci, Macromolecules, 2013, 46, 8773 CrossRef CAS.
- M. W. Wang, C. H. Lin and T. Y. Juang, Macromolecules, 2013, 46, 8853 CrossRef CAS.
- K. D. Demir, B. Kiskan, S. S. Latthe, A. L. Demirel and Y. Yagci, Polym. Chem., 2013, 4, 2106 RSC.
- S. C. Lin, C. S. Wu, J. M. Yeh and Y. L. Liu, Polym. Chem., 2014, 5, 4235 RSC.
- C. Zhang, Y. Zhang, Q. Zhou, H. Ling and Y. Gu, J. Polym. Eng., 2014, 34, 561 CAS.
- Y. S. Ye, Y. J. Huang, F. C. Chang, Z. G. Xue and X. L. Xie, Polym. Chem., 2014, 5, 2863 RSC.
- A. Badshah, M. R. Kessler, Z. Heng, J. H. Zaidi, S. Hameed and A. Hasan, Polym. Chem., 2013, 4, 3617 RSC.
- N. Ramdani, J. Wang, X. Y. He, T. T. Feng, X. D. Xu, W. B. Liu and X. S. Zheng, Mater. Des., 2014, 61, 1 CrossRef CAS PubMed.
- J. Wang, X. Y. He, J. T. Liu, W. B. Liu and L. Yang, Macromol. Chem. Phys., 2013, 214, 617 CrossRef CAS.
- H. Wang, J. Wang, X. Y. He, T. T. Feng, N. Ramdani, M. J. Luan, W. B. Liu and X. D. Xu, RSC Adv., 2014, 4, 64798 RSC.
- M. Baqar, T. Agag, R. Huang, J. Maia, S. Qutubuddin and H. Ishida, Macromolecules, 2012, 45, 8119 CrossRef CAS.
- P. Chutayothin and H. Ishida, Macromolecules, 2010, 43, 4562 CrossRef CAS.
- C. Liu, D. Shen, R. M. Sebastián, J. Marquet and R. Schonfeld, Macromolecules, 2011, 44, 4616 CrossRef CAS.
- J. Dunkers and H. Ishida, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 1913 CrossRef CAS.
- R. Andreu, J. Reina and J. Ronda, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 6091 CrossRef CAS.
- A. Sudo, S. Hirayama and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 479 CrossRef CAS.
- A. Sudo, A. Mori and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 2183 CrossRef CAS.
- W. J. Burke, J. L. Bishop, E. L. M. Glennie and W. N. Bauer, J. Org. Chem., 1965, 30, 3423 CrossRef CAS.
- H. Oie, A. Mori, A. Sudo and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4756 CrossRef CAS.
- H. Oie, A. Mori, A. Sudo and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 3867 CrossRef CAS.
- G. R. Goward, D. Sebastiani, I. Schnell, H. W. Spiess, H. D. Kim and H. Ishida, J. Am. Chem. Soc., 2003, 125, 5792 CrossRef CAS PubMed.
- A. Arnebold, O. Schorsch, J. Stelten and A. Hartwig, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 1693 CrossRef CAS.
- A. Musa, B. Kiskan and Y. Yagci, Polymer, 2014, 55, 5550 CrossRef CAS PubMed.
- A. W. Kawaguchi, A. Sudo and E. Takeshi, ACS Macro Lett., 2013, 2, 1 CrossRef CAS.
- Z. Beyazkilic, M. U. Kahveci, B. Aydogan, B. Kiskan and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4029 CrossRef CAS.
- J. Narayanan, M. J. Jungman and D. L. Patton, React. Funct. Polym., 2012, 72, 799 CrossRef CAS PubMed.
- J. Bai, Z. Shi, J. Yin and M. Tian, Macromolecules, 2014, 47, 2964 CrossRef CAS.
- S. K. Kim, S. W. Choi, W. S. Jeon, J. O. Park, T. Ko, H. Chang and J. C. Lee, Macromolecules, 2012, 45, 1438 CrossRef CAS.
- K. Zhang, R. Cai, Q. Zhuang, X. Liu, G. Yang and Z. Han, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 1514 CrossRef CAS.
- K. Zhang, R. Cai, Q. Zhuang, X. Liu, G. Yang and Z. Han, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 1514 CrossRef CAS.
- A. A. Alhwaige, T. Agag, H. Ishida and S. Qutubuddin, Biomacromolecules, 2013, 14, 1806 CrossRef CAS PubMed.
- H. Ishida, in Handbook of benzoxazine resins, ed. H. Ishida and T. Agag, Elsevier, Amsterdam, 2011, p. 3 Search PubMed.
- H. E. Kissinger, Anal. Chem., 1957, 29, 1702 CrossRef CAS.
- R. Rogers and E. Morris Jr, Anal. Chem., 1966, 38, 412 CrossRef CAS.
- C. F. Wang, C. H. Zhao, J. Q. Sun, S. Q. Huang, X. D. Liu and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2016 CrossRef CAS.
- C. F. Wang, J. Q. Sun, X. D. Liu, A. Sudo and T. Endo, Green Chem., 2012, 14, 2799 RSC.
- A. W. Kawaguchi, A. Sudo and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 1448 CrossRef CAS.
- A. W. Kawaguchi, A. Sudo and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2523 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra16582a |
| This journal is © The Royal Society of Chemistry 2015 |