Interactions of cationic trimeric, gemini and monomeric surfactants with trianionic curcumin in aqueous solution†
Abstract
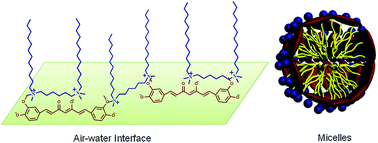
Interactions of trianionic curcumin (Cur3−) with a series of cationic surfactants, monomeric surfactant dodecyl trimethylammonium bromide (DTAB), dimeric surfactant hexamethylene-1,6-bis(dodecyldimethylammonium bromide) (12–6–12) and trimeric surfactant tri(dodecyldimethylammonioacetoxy)diethyltriamine trichloride (DTAD), have been investigated in aqueous solution of pH 13.0. Surface tension and spectral measurements indicate that the cationic surfactants display a similar surfactant concentration dependent interaction process with Cur3−, involving three interaction stages. At first the three cationic surfactants electrostatically bind on Cur3− to form the surfactant–Cur3− complex. Then the bound and unbound cationic surfactants with Cur3− aggregate into surfactant–Cur3− mixed micelles through hydrophobic interactions above the critical micelle concentration of the surfactants (CMCC) in the presence of Cur3−. Finally excess unbound surfactants self-assemble into micelles like those without Cur3−. For all the three surfactants, the addition of Cur3− only decreases the critical micelle concentration of 12–6–12 but does not affect the critical micelle concentration of DTAB and DTAD. As the oligomeric degree of surfactants increases, the intermolecular interaction of the cationic surfactants with Cur3− increases and the surfactant amount needed for Cur3− encapsulation decreases. Compared with 12–6–12, either the weaker interaction of DTAB with Cur3− or stronger interaction of DTAD with Cur3− limits the stability or solubility of Cur3− in surfactant micelles. Therefore, gemini surfactant 12–6–12 is the best choice to effectively suppress Cur3− degradation at very low concentrations. Isothermal titration microcalorimetry, surface tension and 1H NMR results reveal that 12–6–12 and Cur3− form a (12–6–12)2–Cur3− complex and start to form micelles at extremely decreased concentrations, where either 12–6–12 or Cur3− works as a bridge linkage and the resultant structure exhibits the characteristics of oligomeric surfactants.

 Please wait while we load your content...
Please wait while we load your content...