Bifunctional TiO2/Ag3PO4/graphene composites with superior visible light photocatalytic performance and synergistic inactivation of bacteria†
Xiaofei Yang*,
Jieling Qin,
Yan Jiang,
Rong Li,
Yang Li and
Hua Tang*
School of Materials Science and Engineering, Jiangsu University, Zhenjiang 212013, China. E-mail: xyang@mail.ujs.edu.cn; tanghua@mail.ujs.edu.cn; Fax: +86-511-88791947; Tel: +86-511-88790191
First published on 27th March 2014
Abstract
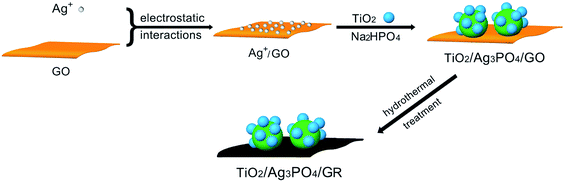
In this work, bifunctional TiO2/Ag3PO4/graphene (GR) composites have been prepared via the combination of ion-exchange method and hydrothermal approach, and the fabrication of “pizza-like” three-phase TiO2/Ag3PO4/GR composites has been achieved through the electrostatic-driven assembly of positively-charged Ag+ on negatively-charged graphene oxide (GO) sheets, followed by the nucleation & controlled growth of Ag3PO4 and the deposition of Degussa P25 on the GO surface. Consequently, the hydrothermal treatment leads to the generation of TiO2/Ag3PO4/GR composites with well-defined structures. The as-prepared composites exhibited highly efficient visible light photocatalytic activity toward organic dye molecule degradation and showed excellent bactericidal performance. This is the first report on the production of bifunctional three-phase metal oxide–Ag3PO4–GR composite materials with improved photocatalytic and antibacterial properties. The improved photocatalytic activity is attributed to the effective separation of photoexcited electron–hole pairs and fast charge transfer between components in the composite, while its excellent bactericidal performance is believed to come from intrinsic bacterial inactivation of Ag3PO4 and photo-induced antibacterial activity of active oxygen-containing radicals generated in the irradiated system. The proper molar ratio of Ag3PO4/TiO2 and the added amount of GO in the precursor have been considered to play crucial roles in the formation of bifunctional composites with promising properties. The TiO2/Ag3PO4/GR composite significantly decreases the percentage of expensive Ag-containing material while it reveals better photocatalytic and antibacterial performance than Ag3PO4, providing new insights into the low-cost, large-scale production of Ag3PO4-based function materials for practical applications.
1. Introduction
The past few decades have witnessed increasing environmental pollution, and exposures to environmental pollution remain a major source of health risk throughout the world. However, environmental pollution cannot be simply generated, and pollutants take many forms. They include not only inorganic and organic chemicals, but also bacteria and organisms. Despite the major efforts that have been made over the past few years to clean the environment, the search for new materials and technology to meet present demands in the removal and sterilization of a wide range of pollutants in water becomes even more urgent. Photocatalysis is an effective, economical and environment-friendly photooxidation process where the produced active oxygen-containing radicals are widely used to remove the contaminants by converting them to CO2, H2O,1–5 etc. Recent advances in new photocatalytic materials and nanotechnology have demonstrated that highly efficient solar photocatalytic degradation of organic pollutants and bacteria in the presence of photocatalysts can be achieved. As a result, the design of highly active visible-light-responsive photocatalytic materials has attracted great attention and a range of visible light photocatalysts have been developed for the environmental decontamination.6–13Most recently, the pioneering work reported by Ye and co-workers has shown that silver orthophosphate (Ag3PO4) can achieve a quantum efficiency of nearly 90% for O2 evolution from water under visible light irradiation.14 Moreover, it has been reported that Ag3PO4 demonstrates significant visible-light-driven photocatalytic activity in the degradation of organic pollutants in aqueous solution.15 Compared with several currently known visible light photocatalysts including doped TiO2, BiVO4, and AgX (X = Cl, Br), Ag3PO4 has a significantly higher photocatalytic efficiency and has been considered to be a promising candidate for practical applications due to its superior photooxidative capabilities by utilizing abundant solar light. However, several limitations of the Ag3PO4 photocatalytic system may restrict its practical use in energy and environmental sciences. Firstly, the Ag3PO4 photocatalyst is unstable upon photo-illumination and it is prone to be photoreduced and decomposed to weakly active Ag. The presence of generated black metallic Ag particles in the photocatalytic system would inevitably prevent visible light absorption and decrease its photocatalytic activity. Secondly, the use of a large amount of expensive silver-containing raw material in the present photocatalytic system strongly limits its large-scale production and practical application. Furthermore, Ag3PO4 normally possesses irregular microstructures and is insoluble in most solvents. The morphology, particle size, and specific highly reactive facet have been proved to play major roles in its highly efficient photocatalytic activity. Most recently, considerable efforts have been made to synthesize Ag3PO4 photocatalysts with well-defined morphologies including cubic Ag3PO4 microcrystals,16 hierarchical Ag3PO4 porous microcubes,17 dendritic Ag3PO4,18 concave trisoctahedral Ag3PO4 microcrystals,19 and Ag3PO4 tetrapods20 and to design Ag3PO4-based composite photocatalysts by the combination of Ag3PO4 with different materials including metal oxides (TiO2,21–23 Fe3O4,24 SnO2 (ref. 25)), Ag,26–30 AgX (X = Cl, Br and I),31–33 carbon materials such as graphene oxide,34–36 carbon quantum dots37 and hydroxyapatite.38,39 Despite tremendous efforts, it is still urgent and highly desirable to develop a facile and low-cost process for the large-scale production of Ag3PO4-based photocatalysts with enhanced stability and highly efficient photocatalytic activity.
Degussa P25, a commercially available TiO2 nanomaterial, consists of two crystal forms of approximately 20% rutile and 80% anatase and has been widely used in photocatalytic studies due to its low cost, photocatalytic activity, stability and innocuousness. However, its visible-light-driven photocatalytic efficiency is generally low due to its wide band gap and the fact that it only absorbs lights in the ultraviolet region. Yao and co-workers reported the synthesis of heterostructured Ag3PO4/TiO2 photocatalysts by the deposition of Ag3PO4 nanoparticles onto the P25 surface via an in situ precipitation method,21 and the obtained composite photocatalyst showed significantly improved photocatalytic degradation of organic dyes compared to that of pure Ag3PO4 and TiO2. Better structural stability and recyclability of the photocatalyst under visible light irradiation were also observed.
Chemically derived graphene oxide (GO) with oxygen-containing functional groups has been proven to be a promising candidate for the construction of composite photocatalysts due to its solubility in solvents and its negatively-charged active sites on its high-surface-area sheets. Our previous report and other groups' work34–36,40 confirm that the hybridization of Ag3PO4 with GO sheets not only results in the enhancement in the visible light absorption, but also leads to an improved visible light photocatalytic performance since GO sheets could facilitate charge transfer and suppress the recombination of photo-generated electrons and holes in the photocatalytic system. Our study further demonstrates that the generation of the Ag3PO4/GR composite photocatalyst by the hydrothermal treatment of the Ag3PO4/GO composite causes an obvious increase in its visible light photocatalytic activity.41
In consideration of the facts that improved stability, enhanced photocatalytic performance and low-cost of Ag3PO4 materials are all important factors responsible for stable and highly efficient Ag3PO4-based visible light photocatalysts. Herein, for the first time, we develop an efficient strategy for the fabrication of TiO2/Ag3PO4/GR composites where the presence of TiO2 and GR in the composite may effectively reduce the cost for the preparation of the composite while the hybridization of photocatalytic P25 and Ag3PO4 on highly conductive GR sheets favors the separation of photo-induced electrons and holes as well as charge transfer in the three-phase composite photocatalyst. The morphology, size, and visible-light photocatalytic behavior of the composites are investigated together with their structural and physicochemical properties. Furthermore, the TiO2/Ag3PO4/GR composites are believed to have intrinsic antibacterial activity and enhanced photo-induced inactivation of bacterial cells under visible light irradiation. To the best of our knowledge, this is the first report concerning the fabrication of bifunctional Ag3PO4-based composites with improved visible light photocatalytic performance and enhanced antibacterial activity. The investigation provides a low-cost and effective approach for the large-scale production of Ag3PO4-based functional composite materials for the efficient removal of organic contaminants and bacterial inactivation under visible light irradiation.
2. Experimental section
2.1 Synthesis of samples
All reagents were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China) and used as received without further purification. Graphite oxide was synthesized from natural graphite by a modified Hummers' method with additional KMnO4.42,43 In a typical synthesis, the as-synthesized graphite oxide was first dispersed in distilled water, followed by ultrasonication for several hours to give GO dispersions. Then AgNO3 solution was added into the above GO solution under magnetic stirring. After gentle stirring overnight, the ultrasonicated Degussa P25 aqueous dispersion was added dropwise into the Ag+–GO mixture, and the mixed solution was stirred for a further 30 min, followed by the addition of Na2HPO4 aqueous solution into the P25–Ag+–GO mixture. Upon the addition of Na2HPO4, yellowish-brown product precipitates were formed instantaneously. The reaction solution was then transferred into a 100 mL Teflon-lined stainless steel autoclave and kept in an oven at 180 °C for 24 h. The autoclave was left to cool naturally to room temperature; the obtained precipitate was collected by centrifugation, washed several times with deionized water and absolute ethanol, and dried at 60 °C under vacuum overnight. The reaction conditions for the preparation of hydrothermal composites are shown in Table 1, and the overall synthetic procedure for the generation of TiO2/Ag3PO4/GR composites is illustrated in Scheme 1. Samples S1, S0.8, S0.6, S0.4, and S0.2 represent the samples prepared by using different molar ratios of Ag3PO4/TiO2, while samples S-0, S-5, S-10, S-20, S-50, and S-100 stand for composites obtained in the presence of different GO amounts.| Sample | GO | AgNO3 | P25 | Na2HPO4·7H2O | M (Ag3PO4/TiO2) |
|---|---|---|---|---|---|
| S0 | 20 mg | 9 mmol, 1.53 g | 3 mmol, 0.804 g | ||
| S1 | 20 mg | 9 mmol, 1.53 g | 3 mmol, 0.24 g | 3 mmol, 0.804 g | 1 |
| S0.8 | 20 mg | 9 mmol, 1.53 g | 3.75 mmol, 0.3 g | 3 mmol, 0.804 g | 0.8 |
| S0.6 | 20 mg | 9 mmol, 1.53 g | 5 mmol, 0.4 g | 3 mmol, 0.804 g | 0.6 |
| S0.4 | 20 mg | 9 mmol, 1.53 g | 7.5 mmol, 0.6 g | 3 mmol, 0.804 g | 0.4 |
| S0.2 | 20 mg | 9 mmol, 1.53 g | 15 mmol, 1.2 g | 3 mmol, 0.804 g | 0.2 |
| S-0 | 9 mmol, 1.53 g | 3.75 mmol, 0.3 g | 3 mmol, 0.804 g | 0.8 | |
| S-5 | 5 mg | 9 mmol, 1.53 g | 3.75 mmol, 0.3 g | 3 mmol, 0.804 g | 0.8 |
| S-10 | 10 mg | 9 mmol, 1.53 g | 3.75 mmol, 0.3 g | 3 mmol, 0.804 g | 0.8 |
| S-20 | 20 mg | 9 mmol, 1.53 g | 3.75 mmol, 0.3 g | 3 mmol, 0.804 g | 0.8 |
| S-50 | 50 mg | 9 mmol, 1.53 g | 3.75 mmol, 0.3 g | 3 mmol, 0.804 g | 0.8 |
| S-100 | 100 mg | 9 mmol, 1.53 g | 3.75 mmol, 0.3 g | 3 mmol, 0.804 g | 0.8 |
2.2 Characterization
The morphologies of the as-synthesized products were examined by field-emission scanning electron microscopy (FESEM, JEOL, JSM-7001F), transmission electron microscopy (TEM, JEOL, JEM-2100) and atomic force microscopy (AFM, MFP-3D SA). The phases of the obtained products were collected on a Bruker D8 Advance X-ray diffractometer (Cu Kα radiation, λ = 0.15406 Å) in a 2θ range from 10° to 80° at room temperature. Raman experiments were performed using a DXR spectrometer using the 532 nm laser line, and measurements were made in backscattering geometry. UV-visible diffuse reflectance spectra were recorded within a 200–800 nm wavelength range using a Shimadzu UV2450 spectrometer.2.3 Photocatalytic experiments
The photocatalytic activities of products were valued by the decomposition of organic dyes under visible-light irradiation. The optical system for the photocatalytic reaction was composed of a 350 W Xe lamp and a cut-off filter (λ > 420 nm). Organic dye solutions (100 mL, 10−5 mol L−1) containing 50 mg of samples were put in a sealed glass beaker, first ultrasonicated for 10 min and then stirred in the dark for 30 min to ensure absorption–desorption equilibrium. After visible light illumination, 4 mL of samples were taken out at regular time intervals (2 min) and separated through centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 10 min). The supernatants were analyzed by recording variations of the absorption band maximum in the UV-vis spectra of the dye molecule by using a Lambda 25 UV/Vis spectrophotometer.
000 rpm, 10 min). The supernatants were analyzed by recording variations of the absorption band maximum in the UV-vis spectra of the dye molecule by using a Lambda 25 UV/Vis spectrophotometer.
2.4 Evaluation of antibacterial activities of samples
Bacteria were cultivated in nutrient broth at 37 °C for 18 h in a rotary shaker until reaching a stationary growth phase. The as-prepared cells were then resuspended and diluted to the required cell density of around 107 colony-forming units per milliliter (CFU mL−1) with sterilized saline solution (0.9% NaCl). The antibacterial activity of the composites was tested on six bacterial strains: Escherichia coli, Staphylococcus aureus, Salmonella typhi, Pseudomonas aeruginosa, Bacillus subtilis, and Bacillus pumilus from ATCC. All of the bactericidal experiments were performed at room temperature and repeated three times in order to give an average value; the measured data for each set of experiments were expressed with the mean and standard deviation.The minimum inhibitory concentrations (MIC) of each of the composites were determined against all test strains. Various concentrations (6.25 ppm, 12.5 ppm, 25 ppm, 50 ppm, 100 ppm, and 200 ppm) of the composites were mixed with freshly sterilized (121 °C, 15 min) nutrient broth (tempered at 37 °C) in glass tubes (15 mm × 15 mm), and consequently 100 μL of pre-cultured strain (initial concentration 106 CFU mL−1) was added into each tube by micropipette. All the tubes were then placed in a precision constant temperature incubator at 35 °C for 48 h where a high-pressure mercury lamp (Philips HPR 125 W) was used as light source. MIC was determined as the lowest composite concentration that resulted in complete inhibition in nutrient broth. For the minimum bactericidal concentration (MBC), the mixtures of a series of concentrations (MIC, 2MIC, 4MIC, …) of composite dispersions with test strains were drawn with one loop full streak-inoculated to the nutrient agar plates. All the plates were then placed in the same incubator equipped with the same light source at 35 °C for 48 h. MBC was determined as the lowest composite concentration that appeared without any colonies observed on the plates.
In order to further investigate the effect of the antibacterial composite on the bacteria cells, the best sample S0.8 was chosen as the best antibacterial composite from the MIC and MBC results and the colonies were counted to determine the viable bacterial numbers after being incubated. In a typical process, PBS buffer was first prepared from a mixture of 0.2 M NaH2PO4 and 0.2 M Na2HPO4 aqueous solutions. S0.8 dispersion with the concentration of 200 ppm was then mixed with sterilized PBS solution, followed by the addition of pre-cultured strain to reach the cell concentration of 106 CFU mL−1. All the tubes were then incubated in a temperature-controlled rotary shaker at 20 °C for different times (0, 0.5 h, 1 h, 2 h, 4 h, and 8 h). For TEM characterizations of untreated and treated samples, 10 μL of each specimen dispersion was first loaded on TEM copper grids and then stained with tungstophosphoric acid aqueous solution. The air-dried copper grids were examined using the TEM (JEOL JEM-2100) as described earlier.
3. Results and discussion
The morphological features of TiO2/Ag3PO4/GR composites were evaluated by SEM, TEM and HRTEM characterization and the results are shown in Fig. 1. SEM observations shown in Fig. 1a and b reveal the formation of pizza-like composite structures. The agglomeration of micro-sized Ag3PO4 particles and TiO2 nanoparticles was observed on the surface of graphene sheets. Two distinct particles were found in the high-magnification SEM image of the composite shown in Fig. 1b. Larger particles ranging from 0.3 μm to 0.8 μm are assigned to as-prepared Ag3PO4 crystals and smaller nanoparticles are attributed to hydrothermally treated Degussa P25. Moreover, the EDX pattern of TiO2/Ag3PO4/GR composites was also recorded and shown in Fig. 1b (inset), showing the presence of several signals from Ti, Ag, P, C and O. The corresponding TEM image of the TiO2/Ag3PO4/GR composites was shown in Fig. 1c and S1† where the particle size characteristics of each component are clearly identified. Irregular micro-sized Ag3PO4 particles and smaller TiO2 nanoparticles were found to be covered by a thin graphene sheet. In addition, a HRTEM image was recorded on the selected area of Fig. 1c, and the high-magnification observation shown in Fig. 1d and S1† clearly confirms the presence of thin and wrinkled graphene layers. The interplanar spacing of 0.35 nm was clearly determined, which corresponds to the (101) crystallographic plane of TiO2, in good agreement with anatase TiO2 (JCPDS no. 21-1272).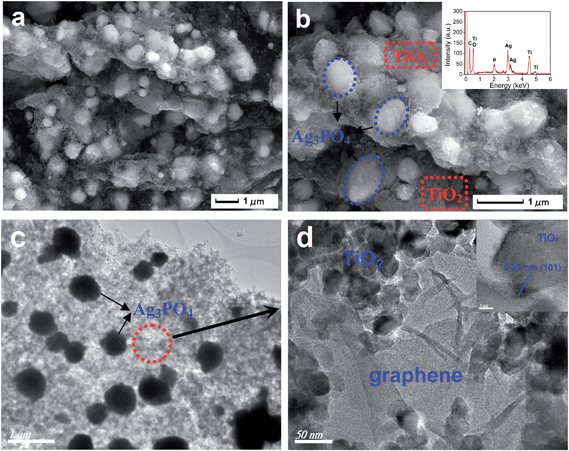 | ||
| Fig. 1 SEM images (a, b, EDX inset) and TEM images (c, d, HRTEM inset) of as-prepared TiO2/Ag3PO4/GR composites. | ||
Fig. 2a shows the XRD pattern of as-synthesized TiO2/Ag3PO4/GR composites. Two diffraction peaks marked by “•” can be readily indexed as the (101) and (211) planes of anatase (JCPDS no. 21-1272), and the rest of the diffraction peaks can be identified to the body-centered cubic phase of Ag3PO4 (JCPDS no. 06-0505). No obvious characteristic diffraction peaks of GR or GO are observed in the XRD pattern of TiO2/Ag3PO4/GR composites. The phenomenon might be ascribed to low diffraction intensities of GR and GO compared to those of crystalline Ag3PO4 and TiO2, as well as the tiny amount graphene oxide employed in the reactants. It is well known that Raman spectroscopy plays an important role in determining the detailed structure of graphitic materials. Thus, Raman spectra of GO, Ag3PO4, and as-synthesized TiO2/Ag3PO4/GR composites were recorded. Two distinct bands at 1355 cm−1 and 1595 cm−1 are observed in the Raman spectrum of GO (Fig. S2a†), corresponding to the D and G bands of graphite, respectively. Several characteristic bands corresponding to the vibrations of Ag3PO4 (Fig. S2b†) appear in the region less than 1200 cm−1, which can be assigned to different modes of Ag3PO4 sample including the external modes, the bending vibration of the tetrahedral PO4 ionic group, and the symmetric stretch of P–O–P and O–P–O bonds. It can be seen from Fig. 2b that the spectrum of the composite also exhibits two peaks at around 1350 cm−1 (D band) and 1590 cm−1 (G band). Moreover, other peaks ranging from 100–1200 cm−1 are believed to come from Ag3PO4 and TiO2. Generally, the intensity ratio of the D and G bands (ID/IG) is applied to evaluate the disorder degree in the graphitic layers and average size of the sp2 domains of the graphitic materials. The ID/IG value of GO is estimated to be about 0.92, while an increased ID/IG value ratio of 1.05 was observed in the TiO2/Ag3PO4/GR composite, indicating that less defects formed in the graphitic layers, which suggests the reduction of GO to GR upon hydrothermal treatment in the composite.
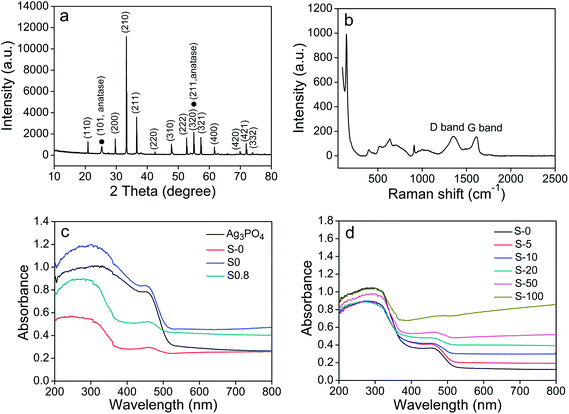 | ||
| Fig. 2 XRD pattern (a) and Raman spectra (b) of TiO2/Ag3PO4/GR hybrids; UV-vis diffuse reflectance spectra of different photocatalysts (c) and different amounts of GO as precursors (d). | ||
It is believed that visible light absorption properties of catalysts are crucial in determining their photocatalytic performance, especially for the photocatalytic pollutant or bacterial degradation under visible light irradiation. Thus diffuse reflectance spectra of different samples were recorded and are shown in Fig. 2c. It is notable that pure Ag3PO4 showed good absorbance in the whole region. The hybridization of Ag3PO4 with P25 (S-0) resulted in the obvious decrease in the absorbance, whereas the introduction of 20 mg GO in precursors (S0) caused a slight increase in the absorbance. The TiO2/Ag3PO4/GR composite S0.8 where 20 mg GO is employed and the molar ratio of Ag3PO4/TiO2 is 0.8 exhibited better absorbance than pure Ag3PO4 in the visible range (500–800 nm) and the composite S-0 in the whole UV-visible range. However, its absorbance is obviously lower than that of S0 sample where 20 mg GO is incorporated with Ag3PO4 in the absence of P25, implying that the introduction of GO into the composite definitely favors the enhanced visible light absorbance while the employment of TiO2 has a negative effect on the visible light absorbance of the composite. It is also shown from Fig. 2d that the presence of different amounts of GO in the precursor affects the optical property of light absorption for the TiO2/Ag3PO4/GR obviously. The added GO has been reduced to GR upon the hydrothermal treatment by losing the majority of functional groups, and the generated GR induces the increased light absorption intensity particularly in the visible region, as observed in all of six composites (S-0, S-5, S-10, S-20, S-50, S-100, and S-200) with different addition amounts of GO. The presence of GR in the composites leads to a continuous absorption band in the visible light region, which is in good agreement with the color of the samples.
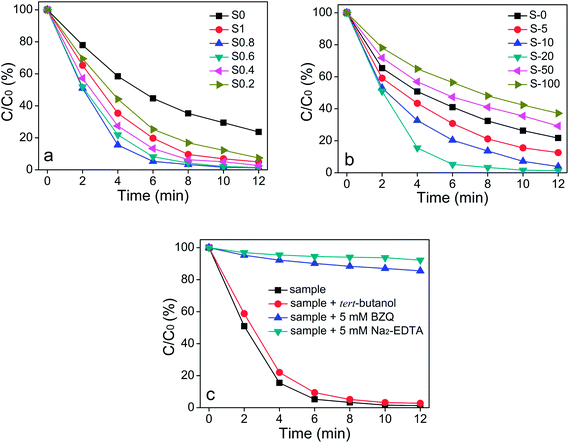
The photocatalytic activities of TiO2/Ag3PO4/GR composites were first evaluated by decomposing RhB under visible light irradiation. It is clearly shown from Fig. 3a that the sample S0 (Ag3PO4/GR composite) exhibits the lowest photocatalytic activity of less than 80% in 12 min. When 0.24 g TiO2 was introduced into the Ag3PO4/GR composite, the corresponding three-phase composite S1 showed enhanced photocatalytic activities of more than 95% in 12 min. Further addition of TiO2 leads to the decrease in the molar ratio of Ag3PO4/TiO2 from 1 to 0.8, resulting in the generation of the TiO2/Ag3PO4/GR composite (S0.8) with the highest photocatalytic activity of around 95% in 6 min and almost 100% in 10 min. However, TiO2/Ag3PO4/GR composites with lower molar ratios of Ag3PO4/TiO2 than 0.8 (S0.6, S0.4, S0.2) showed decreased photocatalytic efficiencies, and all the three-phase TiO2/Ag3PO4/GR composites demonstrate higher photocatalytic performance than the two-phase Ag3PO4/GR composite (S0). The above results imply that the proper molar ratio of Ag3PO4/TiO2 plays a crucial role in determining the photocatalytic activities of TiO2/Ag3PO4/GR composites. Furthermore, when the optimal molar ratio of 0.8Ag3PO4/TiO2 is fixed, the effect of added GO on photocatalytic activities of TiO2/Ag3PO4/GR composites was investigated, and the results are shown in Fig. 3b. The Ag3PO4/TiO2 composite (S-0) reveals the photocatalytic efficiency of around 75% in 12 min in the absence of GO. When 5 mg GO was introduced in the Ag3PO4/TiO2 composite, the photocatalytic activity of the composite S-5 was increased to nearly 85% in 12 min, and a further increase in GO to 10 mg leads to an enhanced photocatalytic activity of more than 95% in 12 min. The highest photocatalytic efficiency of around almost 100% in 10 min was achieved when 20 mg GO was used. However, the addition of a higher amount than 20 mg GO (50 mg, 100 mg) causes a negative effect on the photocatalytic activity of the TiO2/Ag3PO4/GR composite, and the photocatalytic efficiencies of two samples (S-50 and S-100) are even lower than that of the two-phase Ag3PO4/TiO2 composite (S-0), implying the appropriate added GO amount also affects the photocatalytic performance of TiO2/Ag3PO4/GR composites significantly and the best added GO amount in this study is 20 mg.
Furthermore, reactive species trapping experiments were performed to investigate active oxidizing species in the photocatalytic process where three different chemicals, p-benzoquinone (BZQ, a O2−˙ radical scavenger), disodium ethylenediaminetetraacetate (Na2EDTA, a hole scavenger) and tert-butanol (a ˙OH radical scavenger), were employed. The experimental results (Fig. 3c) indicated that when 5 mM Na2–EDTA as well as 5 mM BZQ was introduced to the above photocatalytic system, the photocatalytic activity of the hybrids was intensively suppressed with the degradation efficiency, decreasing from 100% to 20% in 10 minutes. Moreover, the presence of tert-butanol in the TiO2/Ag3PO4/GR photocatalytic system showed negligible effect on its excellent photocatalytic activity. The reactive species trapping results indicate that the photo-induced active holes and superoxide ions make major contributions to the highly efficient photocatalytic performance. In addition to the photocatalytic performance toward RhB, two organic dyes MB and MO were also chosen as model pollutants to evaluate the photocatalytic efficiency of the as-prepared TiO2/Ag3PO4/GR photocatalyst, and the result is shown in Fig. S3.† It can be clearly seen that, when MB was employed, a relatively higher photocatalytic efficiency was observed, while an obvious lower photocatalytic activity was achieved once MO was introduced. It is suggested that the removal of the majority of organic pollutants RhB or MB under visible light irradiation was obtained in 6 min, and a longer irradiation time contributes less to its photocatalytic efficiency. However, the elimination of organic pollutant MO was found to occur gradually at regular intervals of 2 min, achieving a photocatalytic efficiency of around 60% in 6 min and almost 100% in 12 min.
It is well known that Ag-based materials are effective biocides against numerous kinds of bacteria and fungi, and besides, photo-generated oxidative radicals from irradiated TiO2-based materials are capable of inhibiting the growth of bacteria in the photocatalytic process. Thus, it is reasonable to assume that the obtained TiO2/Ag3PO4/GR composite could be a promising candidate for the disinfection of water by synergistic effects from photocatalytic inactivation of microorganisms and direct bacterial inhibition/killing. For the first time, the intrinsic antibacterial and photocatalytic disinfection of different composite materials were investigated, and the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of different samples under light irradiation are shown in Table 2. It is clearly shown that six samples with different molar ratios (Ag3PO4/TiO2) all exhibited excellent bacterial inhibition activities against four different bacteria. MIC values against S. aureus, S. typhi and P. aeruginosa are observed to be lower than 100 ppm, while a relatively higher value of 100 ppm is found against E. coli. The bactericidal activities of the above samples are further confirmed by MBC test results; notably, the majority of bacteria can be completely killed in a concentration equivalent to the MIC value, implying that the bacteria can be killed simultaneously soon after the growth has been inhibited. When the amount of GO was changed in the precursor, where the molar ratio of Ag3PO4/TiO2 was kept at 0.8, six samples still demonstrate excellent antibacterial activities against four bacteria. Similar lower MIC and MBC values against three bacteria, S. aureus, S. typhi and P. aeruginosa, were obtained while E. coli is still an exception. With the increase in the added amount of GO in the precursor from 0 to 5 mg, 10 mg, and 20 mg, the MIC or MBC of the corresponding sample against three bacteria was observed to be further decreased to a lower value, indicating that the introduction of an increased amount of GO causes enhanced bacterial inhibition or bactericidal activity of the sample. However, when more than 20 mg GO was employed in the precursor, as-prepared TiO2/Ag3PO4/GR composites exhibit slightly poorer bacterial inhibition or bactericidal activity toward three bacteria, implying that the presence of a higher percentage of GR reduced from GO upon hydrothermal treatment has a negative effect on the antibacterial activity of the TiO2/Ag3PO4/GR composite. It is generally accepted that the direct bacterial activity of Ag3PO4 should result from dissolved Ag+, and the photocatalytic inactivation of bacteria also makes a major contribution to excellent antibacterial performance of TiO2/Ag3PO4/GR composites. Under visible light irradiation, the synergistic effects from Ag+ and photo-induced oxidative species lead to total and efficient bacterial removal. For the TiO2/Ag3PO4/GR samples prepared from precursors with different amounts of GO, it is proposed that the proper addition of GO in the precursor may improve the solubility/dispersibility of the sample, which favors membrane penetration of the antibacterial composite into the host cell and leads to inactivation of bacteria more efficiently. However, a further increase in the added amount of GO in the precursor results in a higher percentage of reduced GR in the TiO2/Ag3PO4/GR composite, due to the fact that GR only presents limited antibacterial activity. Consequently, the TiO2/Ag3PO4/GR composite with a higher percentage of GR demonstrates decreased bacterial inhibition or bactericidal effects.
| Sample | MIC (MBC) | |||
|---|---|---|---|---|
| E. coli | S. aureus | S. typhi | P. aeruginosa | |
| S0 | 100 (100) | 50 (50) | 6.25 (6.25) | 6.25 (12.5) |
| S1 | 100 (100) | 12.5 (25) | 6.25 (12.5) | 12.5 (12.5) |
| S0.8 | 100 (100) | 6.25 (12.5) | 6.25 (12.5) | 12.5 (12.5) |
| S0.6 | 100 (100) | 12.5 (25) | 12.5 (12.5) | 12.5 (25) |
| S0.4 | 100 (100) | 25 (50) | 25 (25) | 12.5 (50) |
| S0.2 | 100 (100) | 50 (50) | 50 (50) | 25 (50) |
| S-0 | 100 (100) | 50 (100) | 25 (50) | 25 (25) |
| S-5 | 100 (100) | 50 (50) | 25 (25) | 12.5 (25) |
| S-10 | 100 (100) | 25 (12.5) | 12.5 (25) | 12.5 (12.5) |
| S-20 | 100 (100) | 6.25 (12.5) | 6.25 (12.5) | 12.5 (12.5) |
| S-50 | 100 (100) | 25 (25) | 25 (50) | 25 (25) |
| S-100 | 100 (100) | 25 (50) | 50 (50) | 25 (50) |
On the basis of the above photocatalytic and antibacterial results, the TiO2/Ag3PO4/GR sample S0.8 where the added GO was 20 mg and the molar ratio of Ag3PO4/TiO2 was 0.8 has been evaluated as the best three-phase composite with highly efficient photocatalytic performance and excellent antibacterial activities. In order to further understand the synergistic effects of the TiO2/Ag3PO4/GR composite S0.8, control experiments including MIC (MBC) of the composite S0.8 with E. coli in dark, E. coli under the light irradiation, and mixtures of S0.8 with E. coli under the light irradiation were conducted, and the results are shown in Table 3.
| Sample | MIC (MBC) | ||
|---|---|---|---|
| S0.8 with E. coli in dark | E. coli under the light | S0.8 with E. coli under the light | |
| E. coli | >800 (>800) | >800 (>800) | 100 (100) |
| S. aureus | >800 (>800) | >800 (>800) | 6.25 (12.5) |
| S. typhi | >800 (>800) | >800 (>800) | 6.25 (12.5) |
| P. aeruginosa | >800 (>800) | >800 (>800) | 12.5 (12.5) |
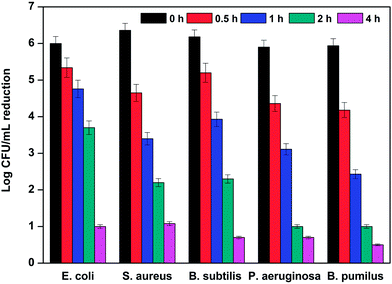
Furthermore, the determination of rapidity and bactericidal duration of the sample S0.8 has been assessed by time-kill analysis. It is shown from Fig. 4 that treatment with 200 ppm aqueous dispersion of S0.8 demonstrated a strong bactericidal effect on different kinds of bacteria. Considerably, within the first 2 h, the bacterial population was observed to decrease dramatically from above 6–6.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU mL−1 of the control to 2.1–2.4
log CFU mL−1 of the control to 2.1–2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU mL−1 for S. aureus and B. subtilis, and around 1.0
log CFU mL−1 for S. aureus and B. subtilis, and around 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU mL−1 for P. aeruginosa and B. pumilus. When the time was prolonged to 4 h, bacterial counts continued to decrease and the number of cells of all bacteria fluctuated around 1
log CFU mL−1 for P. aeruginosa and B. pumilus. When the time was prolonged to 4 h, bacterial counts continued to decrease and the number of cells of all bacteria fluctuated around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU mL−1. After 8 h, the bacterial population was completely inactivated, and the data are not listed in Fig. 4.
log CFU mL−1. After 8 h, the bacterial population was completely inactivated, and the data are not listed in Fig. 4.
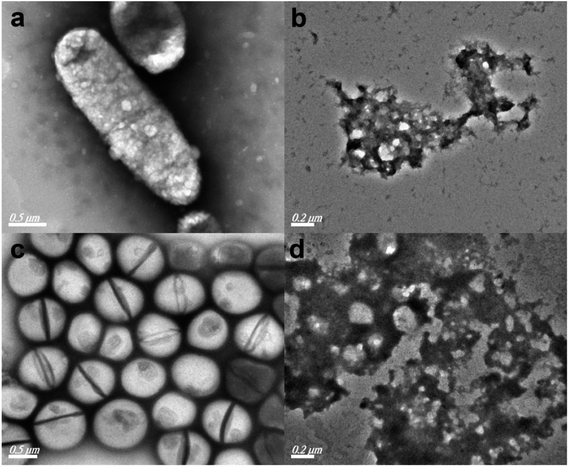
Furthermore, TEM characterization was used to investigate the morphological changes of typical Gram-negative bacteria E. coli and Gram-positive bacteria S. aureus cells before and after the disinfection reaction. As shown in Fig. 5a and c, the E. coli and S. aureus cells remained in a good state in the absence of the antibacterial sample S0.8. However, when the composite was introduced into the system and the composite–bacteria mixture was incubated, it is evident from Fig. 5b and d that obvious cell damages or membrane deformation could be observed for both bacteria, in which regular rod-like or spherical cellular shapes, as well as bigger cell sizes are found to disappear. TEM observations imply that the TiO2/Ag3PO4/GR composite may react with membrane proteins to damage the cell membrane, resulting in the inactivation of its relative function. It is suggested that the addition of GO in the precursor improved the solubility of the composite in antibacterial experiments, and the high-surface-area GR sheet reduced from GO could adsorb and gather the bacteria onto its surface, resulting in enhanced interactions between bacteria and active bactericidal components on GR sheets.
It is reasonably expected that the as-synthesized three-phase TiO2/Ag3PO4/GR composite S0.8 displays highly efficient visible-light-driven photocatalytic activity and demonstrates excellent bactericidal performance against different kinds of bacteria based on the above results and discussion. Due to the presence of graphene and heterogeneous structures, it is necessary to understand the possible mechanism for the photocatalytic decoloration of organic dyes and inactivation of bacteria. As a result, a schematic diagram of the charge separation and transfer in the TiO2/Ag3PO4/GR composite under visible light irradiation is proposed and is shown in Scheme 2. Generally, several factors are responsible for the enhanced photocatalytic decoloration and bactericidal performance. Firstly, the introduction of GO into the precursor plays an important structure-directing role in the generation of well-defined “pizza-like” TiO2/Ag3PO4/GR composites. The presence of negatively-charged GO sheets may absorb positively-charged Ag+ on the large-surface-area GO sheets by the electrostatically-driven assembly. Due to the nature of TiO2 in a dispersion pH greater than 6, when Degussa P25 is added into the Ag+–GO mixture, ultrasonicated TiO2 nanoparticles can be adsorbed onto the Ag+ surface. The subsequent addition of PO43− results in the formation and the controlled growth of Ag3PO4 on the GO surface, suggesting the construction of well-defined three-phase TiO2/Ag3PO4/GO structures. The following hydrothermal treatment at 180 °C for 24 h caused little effect on the structure of the composite where the reduction of GO to GR occurs. Compared with pure Ag3PO4, TiO2 and two-phase TiO2/Ag3PO4 composite, as-prepared TiO2/Ag3PO4/GR composites demonstrate better solubility/dispersibility, which makes a primary contribution to the irradiated photocatalytic process and antibacterial experiments under visible light. Secondly, either GO or GR has a large surface area, and the TiO2/Ag3PO4/GR composite exhibits higher adsorption capacity of organic dyes than Ag3PO4, TiO2 and two-phase TiO2/Ag3PO4 composite. Moreover, the presence of black GR in the TiO2/Ag3PO4/GR composite enhances the absorbance in the visible light region as shown in Fig. 2d. Both the improved adsorption of pollutant/bacteria and effective visible light utilization are helpful for the enhanced photocatalytic performance. Furthermore, most importantly, the formation of the heterostructures is believed to play key roles in the highly efficient photocatalytic performance and antibacterial activities. It is well-known that an effective charge separation/transfer is crucial for the enhancement in photocatalytic activities. For the TiO2/Ag3PO4/GR composite, potentials of both the conduction band and valence band of TiO2 are more negative than those of Ag3PO4 (conduction band: 0.45 eV, valence band: 2.45 eV). Under visible light irradiation, TiO2 nanoparticles possess a large band gap of 3.0 eV that cannot absorb visible light under the present conditions with filtered λ > 420 nm, while the valence band (VB) and the conduction band (CB) of Ag3PO4 can be separated easily. Due to the presence of conductive GR sheets, it can serve as an effective acceptor of the photoexcited electrons. Hence, the photogenerated CB electrons of Ag3PO4 can be transferred to GR sheets in the TiO2/Ag3PO4/GR composite. The transportation and mobility of electrons on GR sheets is very rapid in the specific π-conjugated structure, and thus, the efficient electron transfer from Ag3PO4 to GR sheets keeps electrons away from the Ag3PO4. More photo-generated electrons and holes are produced by continuously working in this way, effectively suppressing the charge recombination and improving the photocatalytic activity. Meanwhile, well-separated electrons in GR sheets can be trapped by the absorbed oxygen in GR surface to generate reactive oxygen species (ROSs), such as superoxide anions (O2−), and the produced active radical species can decompose dye molecules into CO2, H2O, etc. and attack specific bacteria. In addition, the photo-induced holes on the surface Ag3PO4 particles may significantly accelerate the photocatalytic degradation of organic dyes or bacteria. It is also confirmed from XRD patterns of different samples before and after recycled photocatalytic experiments in Fig. S3† that the presence of GR in the TiO2/Ag3PO4/GR composite effectively protects Ag3PO4 from being decomposed into metallic Ag, suggesting better stability and recyclability of TiO2/Ag3PO4/GR composite in the photocatalytic process.
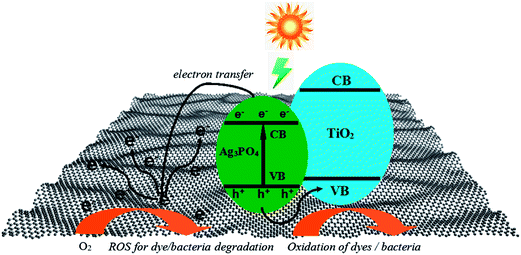 | ||
| Scheme 2 Diagram of the mechanism of photocatalytic degradation of organic dye molecules and bacteria under visible light irradiation. | ||
4. Conclusion
In summary, we have demonstrated an effective hydrothermal approach for the fabrication of TiO2/Ag3PO4/GR composites. The TiO2/Ag3PO4/GR composite shows highly efficient photocatalytic degradation activity toward organic dye molecules and also exhibits excellent antibacterial activity against common bacteria. Bifunctional TiO2/Ag3PO4/GR composites illustrate improved visible light photocatalytic performance and enhanced antibacterial activity compared with bare Ag3PO4, TiO2 and two-phase composites, due to the generation of composite materials. By adjusting the molar ratio of Ag3PO4/TiO2 and the added amount of GO, the photocatalytic and antibacterial activities of the composites can be regulated. As a result, this novel bifunctional TiO2/Ag3PO4/GR composite may find promising applications in environmental protection and water disinfection.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51102116, 11102075, 51302112), Natural Science Foundation of Jiangsu (BK2011480, BK2011534) and Open Fund of Key Laboratory for Intelligent Nano Materials and Devices of the Ministry of Education (INMD-2014M02).References
- J. L. Wang and L. J. Xu, Crit. Rev. Environ. Sci. Technol., 2012, 42, 251 CrossRef CAS
.
- J. C. Sin, S. M. Lam, A. R. Mohamed and K. T. Lee, Int. J. Photoenergy, 2012, 2012, 185159, DOI:10.1155/2012/185159
.
- C. P. Silva, M. Otero and V. Esteves, Environ. Pollut., 2012, 165, 38 CrossRef CAS PubMed
.
- B. Limburg, E. Bouwman and S. Bonnet, Coord. Chem. Rev., 2012, 256, 1451 CrossRef CAS PubMed
.
- S. M. Lam, J. C. Sin, A. Z. Abdullah and A. R. Mohamed, Desalin. Water Treat., 2012, 41, 131 CrossRef CAS
.
- H. Tong, S. X. Ouyang, Y. P. Bi, N. Umezawa, M. Oshikiri and J. H. Ye, Adv. Mater., 2012, 24, 229 CrossRef CAS PubMed
.
- A. Kubacka, M. Fernandez-Garcia and G. Colon, Chem. Rev., 2012, 112, 1555 CrossRef CAS PubMed
.
- Q. J. Xiang, J. G. Yu and M. Jaroniec, Chem. Soc. Rev., 2012, 41, 782 RSC
.
- L. W. Zhang and Y. F. Zhu, Catal. Sci. Technol., 2012, 2, 694 CAS
.
- D. H. Pei and J. F. Luan, Int. J. Photoenergy, 2012, 2012, 262831, DOI:10.1155/2012/185159
.
- A. Di Paola, E. Garcia-Lopez, G. Marci and L. Palmisano, J. Hazard. Mater., 2012, 211, 3 CrossRef PubMed
.
- E. Casbeer, V. K. Sharma and X. Z. Li, Sep. Purif. Technol., 2012, 87, 1 CrossRef CAS PubMed
.
- X. Q. An and J. C. Yu, RSC Adv., 2011, 1, 1426 RSC
.
- Z. G. Yi, J. H. Ye, N. Kikugawa, T. Kako, S. X. Ouyang, H. Stuart-Williams, H. Yang, J. Y. Cao, W. J. Luo, Z. S. Li, Y. Liu and R. L. Withers, Nat. Mater., 2010, 9, 559 CrossRef CAS PubMed
.
- Y. Bi, S. Ouyang, N. Umezawa, J. Cao and J. Ye, J. Am. Chem. Soc., 2011, 133, 6490 CrossRef CAS PubMed
.
- Y. P. Bi, H. Y. Hu, S. X. Ouyang, G. X. Lu, J. Y. Cao and J. H. Ye, Chem. Commun., 2012, 48, 3748 RSC
.
- Q. H. Liang, W. J. Ma, Y. Shi, Z. Li and X. M. Yang, CrystEngComm, 2012, 14, 2966 RSC
.
- Y. P. Bi, H. Y. Hu, Z. B. Jiao, H. C. Yu, G. X. Lu and J. H. Ye, Phys. Chem. Chem. Phys., 2012, 14, 14486 RSC
.
- Z. B. Jiao, Y. Zhang, H. C. Yu, G. X. Lu, J. H. Ye and Y. P. Bi, Chem. Commun., 2013, 49, 636 RSC
.
- J. Wang, F. Teng, M. D. Chen, J. J. Xu, Y. Q. Song and X. L. Zhou, CrystEngComm, 2013, 15, 39 RSC
.
- W. F. Yao, B. Zhang, C. P. Huang, C. Ma, X. L. Song and Q. J. Xu, J. Mater. Chem., 2012, 22, 4050 RSC
.
- R. Y. Liu, P. G. Hu and S. W. Chen, Appl. Surf. Sci., 2012, 258, 9805 CrossRef CAS PubMed
.
- S. B. Rawal, S. D. Sung and W. I. Lee, Catal. Commun., 2012, 17, 131 CrossRef CAS PubMed
.
- G. P. Li and L. Q. Mao, RSC Adv., 2012, 2, 5108 RSC
.
- L. L. Zhang, H. C. Zhang, H. Huang, Y. Liu and Z. H. Kang, New J. Chem., 2012, 36, 1541 RSC
.
- Y. P. Liu, L. Fang, H. D. Lu, L. J. Liu, H. Wang and C. Z. Hu, Catal. Commun., 2012, 17, 200 CrossRef CAS PubMed
.
- Y. P. Liu, L. Fang, H. D. Lu, Y. W. Li, C. Z. Hu and H. G. Yu, Appl. Catal., B, 2012, 115, 245 CrossRef PubMed
.
- Y. P. Bi, H. Y. Hu, S. X. Ouyang, Z. B. Jiao, G. X. Lu and J. H. Ye, J. Mater. Chem., 2012, 22, 14847 RSC
.
- Y. P. Bi, H. Y. Hu, S. X. Ouyang, Z. B. Jiao, G. X. Lu and J. H. Ye, Chem.–Eur. J., 2012, 18, 14272 CrossRef CAS PubMed
.
- W. Teng, X. Y. Li, Q. D. Zhao, J. J. Zhao and D. K. Zhang, Appl. Catal., B, 2012, 125, 538 CrossRef CAS PubMed
.
- Y. Hou, F. Zuo, Q. Ma, C. Wang, L. Bartels and P. Y. Feng, J. Phys. Chem. C, 2012, 116, 20132 CAS
.
- J. Cao, B. D. Luo, H. L. Lin, B. Y. Xu and S. F. Chen, J. Hazard. Mater., 2012, 217, 107 CrossRef PubMed
.
- Y. P. Bi, S. X. Ouyang, J. Y. Cao and J. H. Ye, Phys. Chem. Chem. Phys., 2011, 13, 10071 RSC
.
- Q. H. Liang, Y. Shi, W. J. Ma, Z. Li and X. M. Yang, Phys. Chem. Chem. Phys., 2012, 14, 15657 RSC
.
- L. Liu, J. C. Liu and D. D. Sun, Catal. Sci. Technol., 2012, 2, 2525 CAS
.
- G. D. Chen, M. Sun, Q. Wei, Y. F. Zhang, B. C. Zhu and B. Du, J. Hazard. Mater., 2013, 244–245, 86 CrossRef CAS PubMed
.
- H. C. Zhang, H. Huang, H. Ming, H. T. Li, L. L. Zhang, Y. Liu and Z. H. Kang, J. Mater. Chem., 2012, 22, 10501 RSC
.
- X. T. Hong, X. H. Wu, Q. Y. Zhang, M. F. Xiao, G. L. Yang, M. R. Qiu and G. C. Han, Appl. Surf. Sci., 2012, 258, 4801 CrossRef CAS PubMed
.
- J. J. Buckley, A. F. Lee, L. Olivi and K. Wilson, J. Mater. Chem., 2010, 20, 8056 RSC
.
- H. Y. Cui, X. F. Yang, Q. X. Gao, H. Liu, Y. Li, H. Tang, R. X. Zhang, J. L. Qin and X. H. Yan, Mater. Lett., 2013, 93, 28 CrossRef CAS PubMed
.
- X. F. Yang, H. Y. Cui, Y. Li, J. L. Qin, R. X. Zhang and H. Tang, ACS Catal., 2013, 3, 363 CrossRef CAS
.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806 CrossRef CAS PubMed
.
- X. F. Yang, H. Y. Ding, D. Zhang, X. H. Yan, C. Y. Lu, J. L. Qin, R. X. Zhang, H. Tang and H. J. Song, Cryst. Res. Technol., 2011, 11, 1195 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra01559b |
| This journal is © The Royal Society of Chemistry 2014 |