Supramolecular aggregates between carboxylate anions and an octaaza macrocyclic receptor
Abstract
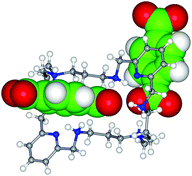
The 28-membered octaazamacrocycle Me2[28]py2N6 was used as a receptor for the molecular recognition of aromatic and aliphatic carboxylate substrates. The receptor-substrate binding behaviour of (H6Me2[28]py2N6)6+ with an aliphatic (−O2C(CH2)nCO2−, n = 0 to 4) and an aromatic (phthalate, isophthalate, terephthalate, 4,4′-dibenzoate, benzoate, 3- and 4-nitrobenzoate) series of carboxylate anions was evaluated by 1H NMR spectroscopy (carried out in DMSO-d6 at 300 K). Two association constants were found for most of the studied cases, except for 3- and 4-nitrobenzoate for which only K1 was determined. For oxalate, malonate, benzoate and dibenzoate anions only the β2 constants could be obtained. The values of the first association constant cover a range from 2.86 to 3.69 (log units), and the second stepwise constant from 2.15 to 2.89 (also in log units). No special selectivity was found but the highest values were determined for adipate and the lowest for the monoprotic 3- and 4-nitrobenzoates. Single crystal X-ray structures of H6Me2[28]py2N66+ with terephthalate, 1, and 4,4′-dibenzoate (2) were determined showing supramolecular entities with general formula (H6Me2[28]py2N6)·(substrate)2(PF6)2·4H2O. These anions are the building blocks of an extensive 3-D network of hydrogen bonds.

 Please wait while we load your content...
Please wait while we load your content...