Open Access Article
Open Access ArticleEco design for Ag-based solutions against SARS-CoV-2 and E. coli†
Anna Luisa
Costa
 *a,
Magda
Blosi
*a,
Magda
Blosi
 *a,
Andrea
Brigliadori
*a,
Andrea
Brigliadori
 a,
Ilaria
Zanoni
a,
Simona
Ortelli
a,
Ilaria
Zanoni
a,
Simona
Ortelli
 a,
Felice Carlo
Simeone
a,
Felice Carlo
Simeone
 a,
Serena
Delbue
b,
Sarah
D'Alessandro
c,
Silvia
Parapini
d,
Claudia
Vineis
e,
Alessio
Varesano
a,
Serena
Delbue
b,
Sarah
D'Alessandro
c,
Silvia
Parapini
d,
Claudia
Vineis
e,
Alessio
Varesano
 e,
Muhammet S.
Toprak
e,
Muhammet S.
Toprak
 f,
Bejan
Hamawandi
f and
Davide
Gardini
a
f,
Bejan
Hamawandi
f and
Davide
Gardini
a
aNational Research Council of Italy, Institute of Science and Technology for Ceramics (CNR-ISTEC), Via Granarolo 64, 48018 Faenza, (RA), Italy. E-mail: anna.costa@istec.cnr.it; magda.blosi@istec.cnr.it
bDepartment of Biomedical, Surgical and Dental Sciences, University of Milan, Via Pascal 36, 20133 Milano, Italy
cDepartment of Pharmacological and Biomolecular Sciences, University of Milan, Via Balzaretti 9, 20133 Milano, Italy
dDepartment of Biomedical Sciences for Health, University of Milan, Via Pascal 36, 20133 Milano, Italy
eNational Research Council of Italy, Institute of Intelligent Industrial Technologies and Systems for Advanced Manufacturing (CNR-STIIMA), Corso Pella 16, 13900 Biella, Italy
fDepartment of Applied Physics, KTH Royal Institute of Technology, SE106 91 Stockholm, Sweden
First published on 17th October 2022
Abstract
For the first time, we exploited the antiviral and antibacterial properties of Ag NPs stabilised by quaternized hydroxyethyl cellulose (Ag-HEC) against SARS-CoV-2 and Escherichia coli through an eco-friendly process at room temperature in three different environments: 1) water, where Ag was dispersed as a nanosol, 2) textiles, where Ag was applied as a coating, and 3) hydrogel where Ag is embedded. The antiviral performance of Ag-HEC nanosols was quantified through the selectivity index (SI), defined as the ratio between 50% cytotoxic and inhibitory concentration, in order to evaluate the ability to be active in a concentration range below the cytotoxicity value. The collected results pointed out an actual enhanced risk/benefit profile of Ag-HEC NPs with respect to chloroquine, with an SI of 22.2 and 8.4, respectively. Antibacterial and antiviral activities of Ag-HEC NPs immobilized on textiles or mucosa-like hydrogels were also assessed and their efficacy in potential application as protective clothing or nasal molecular masks was verified. This work demonstrated that a modern, safe and sustainable design allows traditional colloidal silver-based technologies to be efficiently exploited for a broad spectrum of antimicrobial solutions against bacterial and viral infections.
Environmental significanceAntimicrobial nano silver-based solutions have been investigated since ancient times, but more than ever need reliable and sound strategies for their eco design and verification. Our easily scalable synthetic route, transforming benign reagents, at room temperature, with almost complete yield, matches sustainability criteria. The produced AgHEC nanosol tested against SARS-CoV 2 overcomes the big safety challenge of improving selectivity and identifying use conditions where the antimicrobial activity is maximum, and toxicity is below the identified threshold limits (selectivity index > 10). The reduction, or suppression, of bacterial and viral activities of AgHEC, also verified in textile coatings or hydrogel matrices, supports the use of Ag-HEC as an effective antimicrobial agent for the industrial production of protective clothing or nasal molecular masks. |
1. Introduction
The proliferation and spread of resistant microorganisms pose lethal threats to humans and animals. Just in the last two decades, after the severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV), SARS-CoV-2 emerged as the third highly pathogenic human coronavirus; SARS-CoV-2 alone has caused the recent COVID-19 disease global pandemic.1 Protection from these viruses and bacteria entails two complementary strategies: 1) the inhibition of the development of the diseases that follow infections, and 2) the prevention of the infection itself by minimizing contact with active pathogens. Vaccination and antiviral therapies are considered optimal approaches to minimize the consequences of viral infections; these pharmacological solutions, however, cannot guarantee long-term immunity due to intrinsic uncertainties in their efficacy and to rapid mutations of the pathogens; in addition, unavoidable malfunctions in the massive production and distribution of drugs and vaccines do not always ensure their immediate availability. The inactivation of the pathogens before they can reach humans and animals becomes then crucial to counteract the development of viral and bacterial diseases. On this line, in this paper, we report the antiviral and antibacterial activities of a particular class of Ag nanoparticles stabilised in a cellulose-based matrix2 (Ag-HEC NPs) and define the condition of their highest efficacy in different applications.The antimicrobial activity of metals such as silver, gold and copper has been known for centuries,3–6 and the current emergence of bacteria resistant to antibiotics has spurred renewed interest in these materials;7–10 in particular, nanoparticles composed of these materials have been reported to exhibit extraordinary efficacy against virus infection and replication.11–18 The full exploitation of metal nanoparticles as effective antimicrobial agents, however, faces two challenges: 1) the environmentally sustainable production of massive amounts of nano-enabled antimicrobials, and 2) the control of toxicity that antimicrobial activity may induce. The same unique properties of nanomaterials, in fact, that promise to transform and improve currently available technologies may involve hard-to-predict risks to human health and the environment.19–21 It must be also mentioned that existing nanotechnology solutions suffer from a lack of a clear and scientifically sound quality and efficacy validation pathway that, starting from the design stage of the product development, ensures functionality, safety, sustainability and regulatory compliance (or, preparedness).22 To this purpose, knowledge from EU nano-safety projects can really be helpful for defining decision criteria guiding the selection of the best nanomaterials and manufacturing processes. An example is reported in previous papers,23,24 suggesting a strategy for a safe by design (SbD) use and verification of Ag NP based coatings.
As reported by Costa and Blosi,2 Ag-HEC NPs can be produced by an up-scalable eco-friendly process. The mechanisms of antimicrobial activity of Ag NPs, however, are not yet fully understood; data suggest that differences in structural, physical, and chemical characteristics lead to the observed broad variability of antimicrobial activity of Ag NPs.14,24–29 Among methods of green synthesis of silver nanoparticles, polysaccharides are used as capping agents, or in some cases, can serve as both reducing and capping agents. Some examples of synthesis of cellulose-AgNPs are reported in the literature; nevertheless, they require thermal treatments and work at very low concentration of Ag (lower than two orders of magnitude in comparison with the proposed synthesis).30 The alternative use of a quaternised hydroxyethyl cellulose takes advantage of the presence of ionic ammonium groups that are expected to improve the coordination capacity of cellulose surrounding the as-synthesised Ag-nuclei and to add the intrinsic antimicrobial and adhesive activities of the polymeric quaternary ammonium compounds to the designed antimicrobial nano-phases.31 To assess the use of Ag-HEC, in this paper we focus on the assessment of the antimicrobial activity against SARS-CoV-2 and Escherichia coli in three different forms (representative of potential applications): i) dispersed in water, ii) deposited on fabrics, and iii) embedded in mucose-like hydrogels (3D-hydrogel scaffolds). Results from this analysis will be used to establish the conditions that minimize harmful risks preserving the antiviral and antibacterial activities of the Ag-HEC NP-based substrates.
2. Results and discussion
2.1. Physicochemical characterization
6-month aging does not alter the colloidal stability of the AgHEC nanosol, the sample shows a lower hydrodynamic diameter and polydispersity index (PDI), and is characterized by a lower Ag salt to Ag metal degree of conversion, as the reaction was completed during the storage time (Table 1). In order to characterize the samples also in culture media where the cytotoxicity is measured, we diluted the Ag-HEC nanosol in DMEM + FBS 10%, and verified the expected formation of a negative corona29,34,35 (negative zeta potential), with an increase of the percentage of the ionic fraction at the equilibrium, due to the complexation of metal ions with proteins.36
| Sample | d DLS (nm) | PDI | Zeta potential (mV) | pH | Ag+/Ag tot (%) |
|---|---|---|---|---|---|
| The Ag-HEC nanosol has been diluted in MilliQ water and in culture medium (50 μg mL−1, 1 h of exposure).a 6 months of storage at 4 °C. | |||||
| Fresh Ag-HEC (MilliQ water) | 328 ± 52 | 0.4 | 17.5 ± 2 | 12.2 ± 0.1 | 0.07 ± 0.02 |
| Ageda Ag-HEC (MilliQ water) | 241 ± 9 | 0.3 | 6.3 ± 0.4 | 12.9 ± 1.4 | 0.02 ± 0.01 |
| Ag-HEC (DMEM + FBS 10%) | 93 ± 3 | 0.6 | −1.9 ± 0.5 | 8.5 ± 0.2 | 0.20 ± 0.10 |
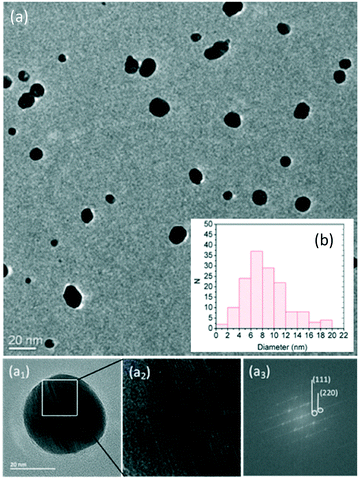
A high degree of crystallinity of the Ag NPs can be revealed from Fourier-transform of TEM images that exhibited, in fact, visible lattice fringes (Fig. 1-a2). Some of the points on the FFT are indexed to cubic Ag lattice planes, which are marked with the corresponding indices on the FFT micrograph (Fig. 1-a3).
| Sample | Wd (g) | Swelling (%) | Dissolution (%) |
|---|---|---|---|
| Ag-HEC/K-Carr | 0.81 | 20.8 | 13.4 |
| Ag-HEC/Agar | 0.42 | 36.5 | 0.1 |
| Ag-HEC/K-Carr/Chit | 0.44 | 32.7 | 7.0 |
| Ag-HEC/K-Carr/HEC | 0.44 | 37.4 | 11.3 |
The K-carrageenan-based hydrogel showed the smallest swelling capacity and the highest degree of dissolution in water, whilst the addition of chitosan or agar improved both the swelling capacity (i.e., the amount of water that the hydrogel can absorb without damage) and the stability in water.
2.2. Cytotoxicity tests
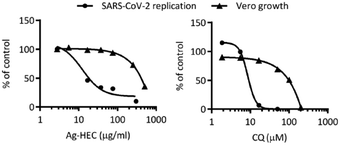
The in vitro cytotoxicity of the Ag-HEC nanosol was investigated by the MTT assay and compared to that of chloroquine (CQ), a well-known antibacterial and virucidal drug. Exposing the Vero cell cultures to a series of Ag-HEC nanosols and CQ at different concentrations yielded values of CC50 and CC10, which are the concentrations that lead to 50% and 10%, respectively, reduction of cell viability (Table 3 and Fig. 3).Based on these data, the lowest cytotoxic concentration of the Ag-HEC nanosol was found to be 126 ± 28 μg mL−1. We observe that this value is well below 1 wt% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg mL−1), that is the maximum limit suggested by the scientific committee on consumer safety (SCCS),38 reported in the latest scientific advice on the safety of nanomaterials and cosmetics; so, considering the hypothesized application and based on the results of cytotoxicity tests, we can estimate concentrations up to 300 μg mL−1 are applicable and can be used to compare the antiviral activity of Ag NPs with chloroquine.
000 μg mL−1), that is the maximum limit suggested by the scientific committee on consumer safety (SCCS),38 reported in the latest scientific advice on the safety of nanomaterials and cosmetics; so, considering the hypothesized application and based on the results of cytotoxicity tests, we can estimate concentrations up to 300 μg mL−1 are applicable and can be used to compare the antiviral activity of Ag NPs with chloroquine.
2.3. Antiviral activity
The second scheme, adopted also for chloroquine, did not lead to any observable effect, while we detected a reduction of viral activity in the first case. These observations suggest that Ag NPs interfere directly with the virus and prevent the contact between active SARS-CoV-2 and the cells.
Table 4 summarizes the results obtained by qRT-PCR expressed in terms of difference in the cycle threshold ΔCt and viral load (as the average of minimum three experiments), and the percentage of SARS-CoV-2 replication. The Ag-HEC nanosol at the highest concentration of 300 μg mL−1 showed an antiviral activity comparable with CQ at 16.7 μM that was tested following the second scheme of exposure ([virus + cells] + CQ). These results reveal different mechanisms of antiviral action for Ag-HEC NPs and CQ; Ag-HEC NPs inactivate the virus before it penetrates into the cells, whilst CQ affects the replication steps once the virus has entered into the host cell. The details of the mechanism of antiviral action of Ag NPs are not yet fully clarified; nevertheless the most accredited hypothesis, consistent with our observations, assumes that, outside the cell, viruses may adsorb on the surface of NPs, which, in turn, mask the receptor and binding domain (spike proteins).39,40 In contrast, CQ can act at the early stage of virus replication, blocking intracellular signaling and affecting virion assembly and budding.41
| Concentration ofAg-HEC nanosol (μg mL−1) | ΔCt | Viral load (copies per μL) | % SARS-CoV-2 replication |
|---|---|---|---|
| Data for the difference in cycle threshold (ΔCt) and viral load are mean values ± standard deviations of, at least, three experiments.a = p < 0.05. | |||
| 300 | −5.03 ± 1.20 | 5.11 × 108a ± 6.95 × 105 | 7.27 |
| 150 | −2.46 ± 0.31 | 2.19 × 109a ± 4.49 × 105 | 31.17 |
| 75 | −2.14 ± 0.33 | 1.64 × 109a ± 4.30 × 105 | 23.35 |
| 37 | −1.96 ± 0.40 | 1.75 × 109a ± 3.78 × 104 | 24.95 |
| 16.7 | −2.43 ± 0.02 | 2.39 × 109a ± 2.25 × 104 | 34.09 |
| 5.5 | −0.31 ± 0.20 | 5.18 × 109 ± 5.12 × 104 | 73.89 |
| 300 (HEC_blank) | 0.17 ± 0.02 | 7.59 × 109 ± 6.01 × 105 | 100 |
| 37.5 (HEC_blank) | −0.21 ± 0.08 | 6.70 × 109 ± 4.40 × 105 | 95.30 |
| Chloroquine (control) 50 μM | −7.90 ± 2.18 | 1.80 × 107a ± 4.62 × 102 | 0.25 |
| Chloroquine (control) 16.7 μM | −4.24 ± 1.04 | 4.89 × 108a ± 2.23 × 104 | 6.90 |
| No treatment | 0 | 7.03 × 109a ± 2.35 × 105 | 100 |
Fig. 2 reports the variation of ΔCt for the Ag-HEC nanosol at different concentrations. It can be noted that the antiviral activity remained approximately constant at concentrations below the CC10 (∼150 μg mL−1) and exhibited an abrupt increase at 300 μg mL−1, a concentration higher than the CC50 (275 μg mL−1) for cytotoxicity. We recall that the cytotoxicity of the Ag-HEC nanosol is partially due to the release of Ag+, which induces the denaturation of proteins that regulate ATP production and DNA replication, but also to the generation of radical oxygen species (ROS) that break down membrane and mitochondrial function.25,42
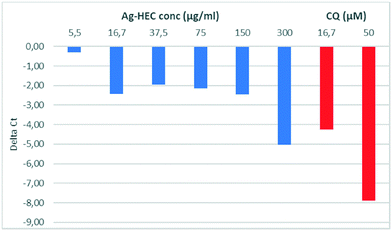 | ||
| Fig. 2 Values of ΔCt for SARS-CoV-2 vs. concentration of Ag-HEC nanosol (blue) and chloroquine (red). | ||
Most likely, at concentrations larger than CC50, a synergistic effect of inactivation of the binding domain of the virus by the Ag NPs43 and cytotoxicity induced by the release of Ag+ occurs and leads to the abrupt increase of antiviral activity around 300 μg mL−1 of Ag-HEC.32
The virucidal activity of the Ag-HEC nanosol was confirmed by the plaque assay in the concentration range of 18 μg mL−1 to 150 μg mL−1 (Table 6), which revealed a reduction of SARS-CoV-2 infectivity by ∼50% at 150 μg mL−1.
The objective of the investigation of cytotoxicity and virucidal activity was to define the concentrations of the Ag-HEC nanosol that can be used in antiviral application with no residual toxicity; this amount can be estimated from the selectivity index (SI), that is, the ratio of cytotoxicity to biological reactivity, in the present study given by CC50/IC50 (the antiviral 50% inhibition concentration). Fig. 3 shows the effect of concentration of the Ag-HEC nanosol and CQ on the SARS-CoV-2 viral load and Vero cell viability, and Table 6 reports the values of the cytotoxicity and antiviral activity and the corresponding values of selectivity index for the Ag-HEC nanosol and chloroquine.
The selectivity index for the Ag-HEC nanosol was found to be above the limit (≥10) assumed for promoting its effective and safe use.44 Furthermore, a selectivity index higher than that of CQ clearly suggested a broader range of safe concentration of the Ag-HEC nanosol to be used in application (Table 5).
| Compound | IC50 | CC50 | CC10 | SI |
|---|---|---|---|---|
| Data are the means ± SD of three independent experiments performed in duplicate; SI = selectivity index = CC50/IC50. | ||||
| Ag-HEC (μg mL−1) | 12.42 | 275.7 ± 64.5 | 126.0 ± 27.8 | 22.2 |
| CQ (μM) | 11.30 | 95.3 ± 18.0 | 20.9 ± 4.4 | 8.43 |
| Mean PFU mL−1 ± SD (% of replication) | |||||
|---|---|---|---|---|---|
| Untreated infected cells | 18 μg mL−1 | 37 μg ML−1 | 75 μg ML−1 | 150 μg ML−1 | |
| a = p < 0.05. | |||||
| Ag-HEC nanosol | 59.38a ± 6.86(100%) | 47.92 ± 6.93(80.7%) | 39.17a ± 5.60(65.97%) | 44.58a ± 6.52(75.09%) | 27.71a ± 3.03(46.67%) |
| Untreated infected cells | 1.9 μM | 5.6 μM | 16.7 μM | 50.0 μM | |
| CQ | 85 (100%) | 73.3 (86.2%) | 54.3 (63.8%) | 0 (0%) | 0 (0%) |
The virus activity on Ag-HEC-loaded fabrics and scaffolds was reduced by about 33% and 44%, respectively, when compared with the untreated substrates, pointing out that although Ag NPs were immobilized on a matrix the antiviral action capability was kept. It is important to stress that withdrawing the virus from the hydrogel scaffolds after exposure was difficult, indicating a potentially synergistic effect against virus replication due to the adsorption/entrapping action of the hydrogel combined with the de-activation by Ag NPs.
These results are also encouraging from the perspective of promoting the safe use of Ag-HEC through the two potential applications suggested by the two nano-enabled products (fabrics to be used in personal protective equipment and hydrogel as a base for protective molecular masks). In fact, as schematized in Fig. S4,† the Ag amount corresponding to the reference dose for oral exposure of bulk Ag, as suggested by the EPA (350 μg person−1 day−1),45 is potentially delivered by a large area of fabrics (from A3 to A1 formats, due to the inhomogeneity of Ag deposited) or by 1/3 of the volume of the hydrogel scaffold produced, so the real dose that can be extrapolated, in the hypothesized condition of use, will be reasonably below such limit.
2.4. Antibacterial activity of Ag-HEC fabrics and scaffolds
The results of antibacterial tests performed on Ag-HEC coated fabrics are reported in Table 8. The tests were performed also after abrasion or washing (laundering cycles) in order to assess washing and mechanical resistance.| Ag-HEC coated fabrics | Bacterial reduction% |
|---|---|
| PVA (reference) | 27.2 ± 1.5 |
| PPNW/PVA/HEC (blank) | 15 ± 1.0 |
| PA 6,6/PVA/HEC (blank) | 15 ± 1.0 |
| PET/PVA/HEC (blank) | 15 ± 1.0 |
| PPNW/PVA/Ag-HEC | 100 |
| PA 6,6/PVA/Ag-HEC | 100 |
| PET/PVA/Ag-HEC | 100 |
| PA 6,6/PVA/Ag-HEC after 1000 abrasion cycles | 95.8 ± 0.9 |
| PA 6,6/PVA/Ag-HEC after 1 laundering | 85.4 ± 1.6 |
| PA 6,6/PVA/Ag-HEC after 10 launderings | 91.2 ± 2.9 |
| PET/PVA/Ag-HEC after 1000 abrasion cycles | 99.0 ± 0.3 |
| PET/PVA/Ag-HEC after 1 laundering | 99.4 ± 0.6 |
| PET/PVA/Ag-HEC after 10 launderings | 84.7 ± 5.4 |
All the Ag-HEC coated fabrics showed a substantial, and in some cases complete, reduction of Escherichia coli after 1 hour. These results revealed also a strong adhesion of Ag NPs to the hydroxyethyl cellulose matrix (HEC) which led to efficient antibacterial activity after washing. Table 9 reports the results for antibacterial activity of the 3D-hydrogel scaffolds, which, by loading with Ag-HEC, exhibited a complete removal of E. coli.
| Sample | Weight (g) | Conc. Ag(wt%) | Bacterial reduction (%) |
|---|---|---|---|
| Blank 1 (K-Carr/HEC/Chit) | 1.08 | 0.00 | 0 |
| Blank 2 (K-Carr/HEC) | 0.94 | 0.00 | 0 |
| Ag-HEC/K-Carr/Chit | 1.12 | 1.07 | 100 |
| Ag-HEC/K-Carr | 1.27 | 1.37 | 100 |
| Ag-HEC/Agar/Chit | 1.10 | 1.07 | 100 |
| Ag-HEC/Agar | 0.87 | 1.37 | 100 |
3. Materials and methods
3.1. Synthesis of Ag-HEC and preparation of the relative nanosol
Ag NPs can be prepared by an efficient (reaction yield, 99.9%) and scalable synthetic protocol patented by Costa and Blosi,46 which leads to a concentrated nanosol (0.1–0.5 wt% Ag) stable for 12 months. In short for Ag-HEC 0.5 wt%, a 0.05 M solution of silver nitrate (Sigma-Aldrich, USA) was added to a solution of quaternized hydroxyethyl cellulose, HEC (Dow Chemical, USA), with a HEC/Ag molar ratio of 5.5; the resulting solution was stirred for 5 minutes. A 1 M solution of NaOH (Sigma-Aldrich, USA) was then added dropwise to the reaction mix up to a NaOH/Ag molar ratio of 2.8 to form a gel. Formation of metal Ag from reduction of Ag ions leads to a black-brown color of the gel, which, however, turned into a yellowish brown low viscosity dispersion after 24 hours.This gel, composed of Ag NPs dispersed in a matrix (network) of quaternized hydroxyethyl cellulose, can be easily dissolved in water to obtain nanosols of suitable concentration; the physicochemical characterization of Ag-HEC nanosols are described in the ESI.†
3.2. Deposition of the Ag-HEC nanosol on different types of fabric and its resistance to washing and abrasion
We used 5 mL of nanosol to deposit, by spray coating, Ag-HEC NPs on textile substrates. To improve the adhesion of Ag-HEC NPs, we added poly(vinyl alcohol), PVA, with a molecular mass of 130 kDa and hydrolysis degree >99% (Sigma-Aldrich, USA), to the nanosol. PVA was dissolved at a concentration of 1.0 wt/vol% in water and kept for 2 hours under stirring at 90 °C. The polymeric solution was cooled down overnight under stirring, then the Ag-HEC nanosol at 0.1 wt% Ag content was added with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio. The resulting PVA/Ag-HEC NPs dispersion was stirred for 2 hours to ensure homogeneity. This dispersion was used to deposit Ag-HEC NPs on three different types of fabric composed of the most used polymeric fibers: 1) spunbonded non-woven polypropylene (PPNW) composed of 16 μm fibers, with an areal density of 23 g m−2; this type of fiber is used in clothing products and in many other applications that include automotive, building products, filtration, and lamination; 2) polyamide (PA 6,6) fabric, with a tread count of 44 × 36 yarns cm−1 and 59 g m−2 areal density; PA 6,6 finds applications in the clothing industry (e.g., fashion, technical, sport, lingerie); and 3) ISO 105 F04 polyethylene terephthalate (PET) fabric; PET yarn is used to produce clothes and carpets. The coated fabrics were then oven dried at 80 °C for 2 min.
1 volume ratio. The resulting PVA/Ag-HEC NPs dispersion was stirred for 2 hours to ensure homogeneity. This dispersion was used to deposit Ag-HEC NPs on three different types of fabric composed of the most used polymeric fibers: 1) spunbonded non-woven polypropylene (PPNW) composed of 16 μm fibers, with an areal density of 23 g m−2; this type of fiber is used in clothing products and in many other applications that include automotive, building products, filtration, and lamination; 2) polyamide (PA 6,6) fabric, with a tread count of 44 × 36 yarns cm−1 and 59 g m−2 areal density; PA 6,6 finds applications in the clothing industry (e.g., fashion, technical, sport, lingerie); and 3) ISO 105 F04 polyethylene terephthalate (PET) fabric; PET yarn is used to produce clothes and carpets. The coated fabrics were then oven dried at 80 °C for 2 min.
We tested the antiviral and antibacterial activities of 20 cm × 20 cm square samples of each coated fabric.
The resistance of the functionalized samples against washing and abrasion was also assessed; washing was assessed, according to ISO 105-C06 A1S, at 40 °C with an ECE detergent and repeated washing cycles of 30 min, while 1000-cycle abrasion tests were performed according to ISO 12947-2 using a Martindale apparatus at 12 kPa loading.
3.3. Embedding of Ag-HEC NPs into hydrogel-based scaffolds
For the preparation of Ag-HEC hydrogel-based scaffolds, the Ag-HEC nanosol (0.5 wt% Ag) was mixed with aqueous solutions of different biopolymers (K-carrageenan, agarose, chitosan) in the amounts reported in Table S1.† The Ag-HEC nanosol was heated up to 90 °C under magnetic stirring and the biopolymeric solutions were added to the Ag-HEC nanosol dropwise and kept at 90 °C for a further 20 min. After that, the suspension was cast into silicon molds and allowed to cool to room temperature for 4 hours. The gelled samples were freeze-dried and easily removed from the molds. 3 cm × 3 cm hydrogel squares (0.5 cm thickness, mass about 0.1 g) were thus prepared. Hydrogels without added Ag were also produced and tested as negative control blanks (Blank 1 and Blank 2). Methods for the determination of the swelling capacity and dissolution in water (stability) of the hydrogels are described in the ESI.†3.4. Cytotoxicity of the Ag-HEC nanosol and comparison with chloroquine
We tested the cytotoxicity of the Ag-HEC nanosol and chloroquine, the reference drug, by the MTT assay in Vero cells (ATCC-CCL81-monkey kidney epithelial cells). Before testing, cells were maintained in DMEM supplemented with 10% heat-inactivated fetal calf serum, 2 mM glutamine, 100 units mL−1 of penicillin, and 100 μg mL−1 of streptomycin. Cytotoxicity was measured on cells seeded into 96-well plates at a concentration of 1 × 104 cells per well. After incubation for 24 hours, cells were treated with serial 4-fold dilutions of the Ag-HEC nanosol (from 500 to 0.5 μg mL−1), or chloroquine (from 200 to 1.6 μM) (tested as positive control), by maintaining a final volume of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μl. After incubation for 72 hours at 37 °C in 5% CO2, cell viability was measured by the MTT assay.47 The absorbance was measured spectrophotometrically at a test wavelength of 550 nm and a reference wavelength of 650 nm, using a Synergy 4 microplate reader (Biotek, GE). The percentage of viable cells was calculated using untreated cells as a control (100% viability) using the formula:
μl. After incubation for 72 hours at 37 °C in 5% CO2, cell viability was measured by the MTT assay.47 The absorbance was measured spectrophotometrically at a test wavelength of 550 nm and a reference wavelength of 650 nm, using a Synergy 4 microplate reader (Biotek, GE). The percentage of viable cells was calculated using untreated cells as a control (100% viability) using the formula: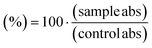 | (1) |
From this equation, we estimated, by the Gene5 software, the CC50, that is, the concentration of Ag-HEC NPs, or chloroquine, that reduced the viability of Vero cells by 50% with respect to the untreated cells. This parameter is very useful in comparing the effects of different compounds on the response of cell culture: in principle, the lower the value of CC50, the more cytotoxic the compound.
3.5. Biological activity
In all the experiments, chloroquine was used as a control drug, although incubated according to a different scheme: after removal of the virus inoculum, the cells were treated with only the medium (control) or the medium containing chloroquine (from 50 to 1.9 μM), and incubated for 72 hours at 37 °C, 5% CO2.
The scheme used for chloroquine was also used with the Ag-HEC nanosol, at the same dose range reported above, in order to check if differences in the mechanism of action occurred by inverting the order of exposure. Therefore, the following two cases were considered for the Ag-HEC nanosol: [Ag-HEC nanosol + virus] + cells (1st case) or [virus + cells] + Ag-HEC nanosol (2nd case).
The antiviral activity of the Ag-HEC nanosol against SARS-CoV-2 was monitored by qRT-PCR.
• The difference in cycle threshold (Ct) values of the supernatant of untreated and treated infected cells (ΔCt = Ct of the supernatant of untreated – Ct of the supernatant treated infected cells); Ct is inversely correlated to the amount of the target; ΔCt = 3 corresponds to an average decrease of viral load of 1 log;48
• The SARS-CoV-2 load expressed as copies per mL;
• The percentage of replication compared to the untreated control calculated based on copies per mL.
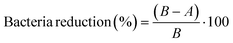 | (2) |
B: number of bacterial colonies in the diluted inoculum.
4. Conclusions
In this study, we presented an effective antimicrobial Ag NP technology prepared by an eco-friendly and easily scalable synthetic route entirely carried out at room temperature in the presence of benign reagents; the method yields Ag NPs embedded in a hydroxyethyl cellulose matrix (Ag-HEC NPs).Antibacterial and antiviral activities were tested against Escherichia coli and SARS-CoV-2 respectively, with the latter one being isolated from a nasal–pharyngeal swab. We proved the antiviral and antibacterial properties of these Ag-HEC NPs in three different forms: dispersed in water (droplet environment), deposited on textile substrates (surface environment), and embedded in a mucose-like hydrogel (biological target environment).
Our findings pointed out an actual enhanced risk/benefit profile of Ag-HEC NPs with respect to chloroquine, as supported by a selectivity index > 10, the recommended limit set for a selective bioactive sample.49
The antiviral activity in the presence of Ag-HEC NPs was set around 30% of SARS-CoV-2 replication passing from very low Ag concentration up to 150 μg mL−1 (below the no-cytotoxicity limit), whilst it abruptly increased at the highest tested concentration (300 μg mL−1), close to the CC50 value, where only 7% of the virus replicated. Unlike what happened with chloroquine, a pre-incubation of Ag-HEC with SARS-CoV-2 is required for efficient inhibition of SARS-CoV-2 replication. This result supports the existence of two distinct antiviral mechanisms: one (for Ag NPs) occurring before virus penetration in the cells, with the second (for CQ), occurring after the virus has entered into the host cell, affecting replication steps.39
The mucose-like hydrogels loaded with Ag NPs showed very promising antibacterial and antiviral properties. In addition to bacteria and virus de-activation, it was observed for the hydrogels a marked sorbent capability which enabled a mechanical entrapment of the virus. Finally, also Ag NPs immobilized on the textile substrate showed the capacity to inhibit virus replication, although a full clarification of the involved mechanism requires further investigations. The reduction, or suppression, of bacterial and viral activities by these different substrates supports the use of Ag-HEC as an effective antimicrobial agent for the industrial production of protective clothing or nasal molecular masks.
Author contributions
Anna Costa: conceptualization, methodology, resources, supervision, writing – original draft, writing – review & editing. Magda Blosi: conceptualization, methodology, resources, supervision, writing – review & editing. Andrea Brigliadori: investigation, methodology, resources, validation, writing – review & editing. Ilaria Zanoni: methodology, resources. Simona Ortelli: resources, validation. Felice Simeone: writing – review & editing. Serena Delbue: investigation, methodology, resources, supervision. Sarah Dalessandro and Silvia Parapini: investigation, methodology. Claudia Vineis and Alessio Varesano: investigation, methodology. Muhammet Toprak and Bejan Hamawandi: investigation, methodology. Davide Gardini: conceptualization, supervision, validation, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We declare that this manuscript has not been published elsewhere in whole or in part and is not under consideration by another journal. Approval of the authors' institution has been granted to publish this work.
General statement: “There are no conflicts to declare”.
Acknowledgements
This work has received funding from the European Union's Horizon 2020 Research and Innovation Programme under Grant Agreement No. 862444 (ASINA – Anticipating Safety Issues at the Design Stage of NAno Product Development) and No. 760928 (BIORIMA - BIOmaterial RIsk MAnagement).References
- Global research on coronavirus disease (COVID-19).
- A. L. Costa and M. Blosi, Process for the preparation of nanoparticles of noble metals in hydrogel and nanoparticles thus obtained, 2016 Search PubMed.
- A. Ebrahiminezhad, M. J. Raee, Z. Manafi, J. A. Sotoodeh and G. Younes, Ancient and Novel Forms of Silver in Medicine and Biomedicine, J. Med. Technol., 2016, 2, 122–128 Search PubMed.
- M. Horue, M. L. Cacicedo, M. A. Fernandez, B. Rodenak-kladniew, R. M. Torres and G. R. Castro, et al. Materials Science & Engineering C Antimicrobial activities of bacterial cellulose – Silver montmorillonite nanocomposites for wound healing, Mater. Sci. Eng., C, 2020, 116, 111152 CrossRef CAS PubMed.
- S. Song, Z. Liu, M. Aamer, L. Ding and J. Zhang, Materials Science & Engineering C Antibacterial polyvinyl alcohol / bacterial cellulose / nano-silver hydrogels that effectively promote wound healing, Mater. Sci. Eng., C, 2021, 126, 112171 CrossRef CAS PubMed.
- R. Vazquez-Munoz and J. L. Lopez-Ribot, Nanotechnology as an Alternative to Reduce the Spread of COVID-19, Challenges, 2020, 11(2), 15 CrossRef.
- S. Behzadinasab, A. Chin, M. Hosseini, L. Poon and W. A. Ducker, A Surface Coating that Rapidly Inactivates SARS-CoV - 2, 2020 Search PubMed.
- B. Balasubramaniam, R. S. Prateek, M. Saraf, P. Kar and S. P. Singh, et al. Antibacterial and Antiviral Functional Materials: Chemistry and Biological Activity toward Tackling COVID-19-like Pandemics, ACS Pharmacol. Transl. Sci., 2021, 4(1), 8–54 CrossRef CAS PubMed.
- J. Hasan, A. Pyke, N. Nair, T. Yarlagadda, K. Spann and P. K. D. V. Yarlagadda, Antiviral Nanostructured Surfaces Reduce the Viability of SARS- CoV - 2, 2020 Search PubMed.
- Y. Wang, H. Tang, D. Wu, D. Liu, Y. Liu and A. Cao, et al. Enhanced bactericidal toxicity of silver nanoparticles by the antibiotic gentamicin, Environ. Sci.: Nano, 2016, 3, 788–798 RSC.
- M. E. Barbinta-Patrascu, N. Badea, C. Pirvu, M. Bacalum, C. Ungureanu and P. L. Nadejde, et al. Multifunctional soft hybrid bio-platforms based on nano-silver and natural compounds, Mater. Sci. Eng., C, 2016, 69, 922–932 CrossRef CAS PubMed.
- E. V. R. Campos, A. E. S. Pereira, O. J. L. De, L. B. Carvalho, M. G. Casagrande and L. R. De, et al. How can nanotechnology help to combat COVID - 19 ? Opportunities and urgent need, J. Nanobiotechnol., 2020, 1–23 Search PubMed.
- L. Chen and J. Liang, An overview of functional nanoparticles as novel emerging antiviral therapeutic agents, Mater. Sci. Eng., C, 2020, 112, 110924 CrossRef CAS PubMed.
- P. Merkl, S. Long, G. M. McInerney and G. A. Sotiriou, Antiviral Activity of Silver, Copper Oxide and Zinc Oxide Nanoparticle Coatings against SARS-CoV-2, Nanomaterials, 2021, 11(5), 1312 CrossRef CAS PubMed.
- G. Pagnotta, G. Graziani, N. Baldini, A. Maso, M. L. Focarete and M. Berni, et al. Nanodecoration of electrospun polymeric fibers with nanostructured silver coatings by ionized jet deposition for antibacterial tissues, Mater. Sci. Eng., C, 2020, 113, 110998 CrossRef CAS PubMed.
- C. Wang, X. Huang, W. Deng, C. Chang, R. Hang and B. Tang, A nano-silver composite based on the ion-exchange response for the intelligent antibacterial applications, Mater. Sci. Eng., C, 2014, 41, 134–141 CrossRef CAS PubMed.
- R. Djellabi, N. Basilico, S. Delbue, S. D. Alessandro, S. Parapini and G. Cerrato, et al. Oxidative Inactivation of SARS-CoV-2 on Photoactive AgNPs @ TiO2 Ceramic Tiles, Int. J. Mol. Sci., 2021, 22, 8836 CrossRef CAS PubMed.
- C. D. Vecitis, Antiviral-nanoparticle interactions and reactions, Environ. Sci.: Nano, 2021, 8(1), 11–19 RSC.
- C. W. Babbitt and E. A. Moore, Sustainable nanomaterials by design, Nat. Nanotechnol., 2018, 13(8), 621–629 CrossRef CAS PubMed.
- S. León-Silva, F. Fernández-Luqueño and F. López-Valdez, Silver Nanoparticles (AgNP) in the Environment: a Review of Potential Risks on Human and Environmental Health, Water, Air, Soil Pollut., 2016, 227(9), 306 CrossRef.
- I. Kieffer, S. Motellier and E. Valsami-jones, Environ. Sci.: Nano, 2021, 806–821 Search PubMed.
- S. Gottardo, A. Mech, J. Drbohlavová, A. Małyska, S. Bøwadt and J. Riego Sintes, et al. Towards safe and sustainable innovation in nanotechnology: State-of-play for smart nanomaterials, NanoImpact, 2021, 21, 100297 CrossRef CAS PubMed.
- D. Gardini, M. Blosi, S. Ortelli, C. Delpivo, O. Bussolati and M. G. Bianchi, et al. Nanosilver: An innovative paradigm to promote its safe and active use, NanoImpact, 2018, 11, 128–135 CrossRef.
- V. Marassi, L. Di Cristo, S. G. J. Smith, S. Ortelli, M. Blosi and A. L. Costa, et al. Silver nanoparticles as a medical device in healthcare settings: A five-step approach for candidate screening of coating agents, R. Soc. Open Sci., 2018, 5(1), 171113 CrossRef PubMed.
- S. P. Deshmukh, S. M. Patil, S. B. Mullani and S. D. Delekar, Mater. Sci. Eng., C, 2019, 97, 954–965 CrossRef CAS PubMed.
- S. Chernousova and M. Epple, Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal, Angew. Chem., Int. Ed., 2013, 52, 1636–1653 CrossRef CAS PubMed.
- T. C. Dakal, A. Kumar, R. S. Majumdar and V. Yadav, Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles, Front. Microbiol., 2016, 7, 1831 Search PubMed.
- L. M. Gilbertson and J. B. Zimmerman, Environ. Sci.: Nano, 2021, 37–66 Search PubMed.
- A. Huk, E. Izak-Nau, B. Reidy, M. Boyles, A. Duschl and I. Lynch, et al. Is the toxic potential of nanosilver dependent on its size?, Part. Fibre Toxicol., 2014, 11, 65 CrossRef PubMed.
- M. A. El-Sheikh, S. M. El-Rafie, E. S. Abdel-Halim and M. H. El-Rafie, Green Synthesis of Hydroxyethyl Cellulose-Stabilized, J. Polym., 2013, 2013, 1–11 Search PubMed.
- J. Shen, B. Li, X. Zhan and L. Wang, A one pot method for preparing an antibacterial superabsorbent hydrogel with a Semi-IPN structure based on tara gum and polyquaternium-7, Polymers, 2018, 10(7), 696 CrossRef PubMed.
- T. T. N. Dung, V. N. Nam, T. T. Nhan, T. T. B. Ngoc, L. Q. Minh and B. T. T. Nga, et al. Silver nanoparticles as potential antiviral agents against African swine fever virus, Mater. Res. Express, 2020, 6(12), 1250g9 CrossRef.
- K. Naik and M. Kowshik, The silver lining: towards the responsible and limited usage of silver, J. Appl. Microbiol., 2017, 123(5), 1068–1087 CrossRef CAS PubMed.
- B. Reidy, A. Haase, A. Luch, K. A. Dawson and I. Lynch, Mechanisms of Silver Nanoparticle Release, Transformation and Toxicity: A Critical Review of Current Knowledge and Recommendations for Future Studies and Applications, Materials, 2013, 6, 2295–2350 CrossRef CAS PubMed.
- I. Lynch and K. A. Dawson, Protein-nanoparticle interactions, Nano Today, 2008, 3(1), 40–47 CrossRef CAS.
- S. Ortelli, A. L. Costa, M. Blosi, A. Brunelli, E. Badetti and A. Bonetto, et al. Colloidal characterization of CuO nanoparticles in biological and environmental media, Environ. Sci.: Nano, 2017, 4(6), 1201–1420 RSC.
- J. D. Torrey, T. L. Kirschling and L. F. Greenlee, Processing and Characterization of Nanoparticle Coatings for Quartz Crystal Microbalance Measurements, J. Res. Natl. Inst. Stand. Technol., 2015, 120, 1–10 CrossRef PubMed.
- *** Scientific Committee on Consumer Safety - SCCS. Scientific Committee on Consumer Safety SCCS - Guidance on the Safety Assessment of Nanomaterials in Cosmetics, 2021, 1–49.
- G. W. Jones, M. P. Monopoli, L. Campagnolo, A. Pietroiusti, L. Tran and B. Fadeel, No small matter: a perspective on nanotechnology-enabled solutions to fight COVID-19, Nanomedicine, 2020, 15(24), 2411–2427 CrossRef CAS PubMed.
- S. Y. Chang, K. Y. Huang, T. L. Chao, H. C. Kao, Y. H. Pang and L. Lu, et al. Nanoparticle composite TPNT1 is effective against SARS-CoV-2 and influenza viruses, Sci. Rep., 2021, 11(1), 1–13 CrossRef PubMed.
- C. A. Devaux, J.-M. Rolain, P. Colson and D. Raoult, New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?, Int. J. Antimicrob. Agents, 2020, 55(5), 105938 CrossRef CAS PubMed.
- S. Galdiero, A. Falanga, M. Vitiello, M. Cantisani, V. Marra and M. Galdiero, Silver Nanoparticles as Potential Antiviral Agents, Molecules, 2011, 16(10), 8894–8918 CrossRef CAS PubMed.
- M. Rai, S. D. Deshmukh, A. P. Ingle, I. R. Gupta, M. Galdiero and S. Galdiero, Metal nanoparticles: The protective nanoshield against virus infection, Crit. Rev. Microbiol., 2016, 42(1), 46–56 CrossRef CAS PubMed.
- L. J. McGaw, E. E. Elgorashi and J. N. Eloff, Cytotoxicity of African Medicinal Plants Against Normal Animal and Human Cells, Toxicol Surv African Med Plants, 2014, pp. 181–233 Search PubMed.
- Washington DCS and RAD, Reference Dose for Chronic Oral Exposure of Silver. CASRN 7440-22-4, US Environ Prot Agency, 1991, pp. 1–13 Search PubMed.
- P. Examiner, Ipnetrcsiatgye, Azpuru CA, 2020, vol. 2 Search PubMed.
- S. D'Alessandro, M. Gelati, N. Basilico, E. A. Parati, R. K. Haynes and D. Taramelli, Differential effects on angiogenesis of two antimalarial compounds, dihydroartemisinin and artemisone: implications for embryotoxicity, Toxicology, 2007, 241(1–2), 66–74 CrossRef PubMed.
- Standard PCR Protocol.
- C. Vonthron-Sénécheau, B. Weniger, M. Ouattara, F. T. Bi, A. Kamenan and A. Lobstein, et al. In vitro antiplasmodial activity and cytotoxicity of ethnobotanically selected Ivorian plants, J. Ethnopharmacol., 2003, 87(2–3), 221–225 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2en00178k |
| This journal is © The Royal Society of Chemistry 2022 |