Design and development of histone deacetylase (HDAC) chemical probes for cell-based profiling†
Abstract
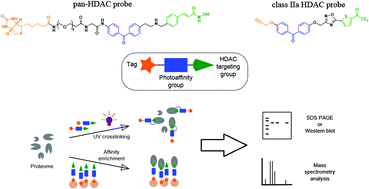
Histone deacetylases (HDACs) contribute to regulation of gene expression by mediating higher-order chromatin structures. They assemble into large multiprotein complexes that regulate activity and specificity. We report the development of small molecule probes with class IIa and pan-HDAC activity that contain photoreactive crosslinking groups and either a biotin reporter, or a terminal alkyne handle for subsequent bioorthogonal ligation. The probes retained inhibitory activity against recombinant HDAC proteins and caused an accumulation of acetylated histone and tubulin following cell treatment. The versatility of the probes has been demonstrated by their ability to photoaffinity modify HDAC targets in vitro. An affinity enrichment probe was used in conjunction with mass spectrometry proteomics to isolate HDACs and their interacting proteins in a native proteome. The performance of the probes in recombinant versus cell-based systems highlights issues for the development of chemoproteomic technologies targeting class IIa HDACs in particular.
- This article is part of the themed collections: Chemical Biology in Molecular BioSystems and Protein Labelling

 Please wait while we load your content...
Please wait while we load your content...