Thermal degradation mechanism of triangular Ag@SiO2 nanoparticles†
Abstract
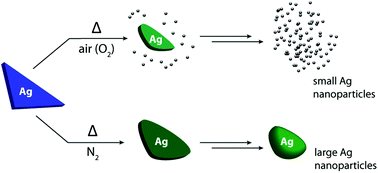
Triangular silver nanoparticles are promising materials for light harvesting applications because of their strong plasmon bands; these absorption bands are highly tunable, and can be varied over the entire visible range based on the particle size. A general concern with these materials is that they are unstable at elevated temperatures. When thermally annealed, they suffer from changes to the particle morphology, which in turn affects their optical properties. Because of this stability issue, these materials cannot be used in applications requiring elevated temperatures. In order to address this problem, it is important to first understand the degradation mechanism. Here, we measure the changes in particle morphology, oxidation state, and coordination environment of Ag@SiO2 nanotriangles caused by thermal annealing. UV-vis spectroscopy and TEM reveal that upon annealing the Ag@SiO2 nanotriangles in air, the triangular cores are truncated and smaller nanoparticles are formed. Ag K-edge X-ray absorption spectroscopy (XANES and EXAFS) shows that the small particles consist of Ag(0), and that there is a decrease in the Ag–Ag coordination number with an increase in the annealing temperature. We hypothesize that upon annealing Ag in air, it is first oxidized to AgxO, after which it subsequently decomposes back to well-dispersed Ag(0) nanoparticles. In contrast, when the Ag@SiO2 nanotriangles are annealed in N2, since there is no possibility of oxidation, no small particles are formed. Instead, the triangular core rearranges to form a disc-like shape.
- This article is part of the themed collection: New Talent: Americas

 Please wait while we load your content...
Please wait while we load your content...