Synthesis and spectroscopic characterization of diorganotin(iv) complexes of N′-(4-hydroxypent-3-en-2-ylidene)isonicotinohydrazide: chemotherapeutic potential validation by in vitro interaction studies with DNA/HSA, DFT, molecular docking and cytotoxic activity†
Abstract
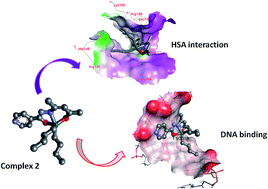
Diorganotin(IV) complexes 1–3 (R = Me, 1; Bu, 2; Ph, 3) derived from the ligand N′-(4-hydroxypent-3-en-2-ylidene)isonicotinohydrazide were synthesized and thoroughly characterized by elemental analysis and spectroscopic techniques (UV-vis, IR, 1H, 13C and 119Sn NMR and ESI-MS). The molecular structure of diphenyltin(IV) complex 3 was further established by single crystal X-ray crystallography which showed that the complex crystallized in the monoclinic space group C21/c. To ascertain the pharmacokinetic and chemotherapeutic aspects of the synthesized diorganotin(IV) complexes 1–3, in vitro interaction studies were carried out with CT DNA/HSA by employing various biophysical methods viz., UV-vis, fluorescence, FT IR (in case of HSA only) and circular dichroism. Notably, all of the complexes exhibited a high propensity for DNA binding via electrostatic modes; the binding affinity was found to be in the order 2 > 3 > 1 and also revealed static quenching of the HSA fluorophore. The experimental findings were validated by density functional theory (DFT) calculations which determined the quantum mechanical (QM) reactivity descriptors viz., single point energy (H), hardness (η), electronic chemical potential (μ), electrophilicity (ω); on that basis the binding trend of the complexes with CT DNA and HSA could be predicted. Further, molecular docking studies were performed to visualize the preferential binding sites of diorganotin(IV) complexes with DNA and HSA. In vitro cytotoxicity of di-n-butyltin(IV) complex 2 was carried out in a panel of human cancer cell lines viz., U373MG (CNS), PC3 (prostrate), Hop62 (lung), HL60 (leukemia), HCT15 (colon), SK-OV-3 (ovarian), HeLa (cervix) and MCF7 (breast) which revealed significantly good activity with GI50 values of <10 μg mL−1 for most of the cell lines tested.

 Please wait while we load your content...
Please wait while we load your content...