SAR studies of 3,14,19-derivatives of andrographolide on anti-proliferative activity to cancer cells and toxicity to zebrafish: an in vitro and in vivo study†
Abstract
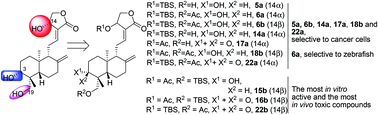
Andrographolide is bestowed with an interesting pharmacophore and has attracted numerous studies on the design and synthesis of andrographolide derivatives. In this study, a small library of 3,14,19-modified derivatives of andrographolide were synthesized and tested for their in vitro inhibitory activities to cancer cell growth and proliferation and in vivo toxicities against zebrafish embryo development. Structure anti-proliferative activity and toxicity relationships in current data revealed that the property of a substituent, substituted positions, and 14-stereochemistry together determined a compound's in vitro anti-proliferative activity against cancer cells, the in vivo toxicity to zebrafish, and the selectivity between MDA-MB-231 and A549. Taken together, our SAR studies discovered some potential leads for further development of anticancer drugs and suggested that the direct and/or indirect toxicity of an active compound with andrographolide pharmacophore should be given attention.

 Please wait while we load your content...
Please wait while we load your content...