Teixobactin analogues reveal enduracididine to be non-essential for highly potent antibacterial activity and lipid II binding†
Abstract
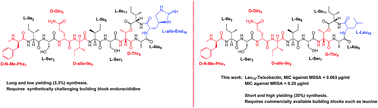
Teixobactin is a highly promising antibacterial depsipeptide consisting of four D-amino acids and a rare L-allo-enduracididine amino acid. L-allo-Enduracididine is reported to be important for the highly potent antibacterial activity of teixobactin. However, it is also a key limiting factor in the development of potent teixobactin analogues due to several synthetic challenges such as it is not commercially available, requires a multistep synthesis, long and repetitive couplings (16–30 hours). Due to all these challenges, the total synthesis of teixobactin is laborious and low yielding (3.3%). In this work, we have identified a unique design and developed a rapid synthesis (10 min μwave assisted coupling per amino acid, 30 min cyclisation) of several highly potent analogues of teixobactin with yields of 10–24% by replacing the L-allo-enduracididine with commercially available non-polar residues such as leucine and isoleucine. Most importantly, the Leu10-teixobactin and Ile10-teixobactin analogues have shown highly potent antibacterial activity against a broader panel of MRSA and Enterococcus faecalis (VRE). Furthermore, these synthetic analogues displayed identical antibacterial activity to natural teixobactin (MIC 0.25 μg mL−1) against MRSA ATCC 33591 despite their simpler design and ease of synthesis. We have confirmed lipid II binding and measured the binding affinities of individual amino acid residues of Ala10-teixobactin towards geranyl pyrophosphate by NMR to understand the nature and strength of binding interactions. Contrary to current understanding, we have shown that a cationic amino acid at position 10 is not essential for target (lipid II) binding and potent antibacterial activity of teixobactin. We thus provide strong evidence contrary to the many assumptions made about the mechanism of action of this exciting new antibiotic. Introduction of a non-cationic residue at position 10 allows for tremendous diversification in the design and synthesis of highly potent teixobactin analogues and lays the foundations for the development of teixobactin analogues as new drug-like molecules to target MRSA and Mycobacterium tuberculosis.


 Please wait while we load your content...
Please wait while we load your content...