Polymerized ionic liquid diblock copolymers: impact of water/ion clustering on ion conductivity†
Abstract
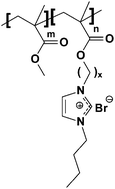
Herein, we examine the synergistic impact of both ion clustering and block copolymer morphology on ion conductivity in two polymerized ionic liquid (PIL) diblock copolymers with similar chemistries but different side alkyl spacer chain lengths (ethyl versus undecyl). When saturated in liquid water, water/ion clusters were observed only in the PIL block copolymer with longer alkyl side chains (undecyl) as evidenced by both small-angle neutron scattering and intermediate-angle X-ray scattering, i.e., water/ion clusters form within the PIL microdomain under these conditions. The resulting bromide ion conductivity in the undecyl sample was higher than the ethyl sample (14.0 mS cm−1versus 6.1 mS cm−1 at 50 °C in liquid water) even though both samples had the same block copolymer morphology (lamellar) and the undecyl sample had a lower ion exchange capacity (0.9 meq g−1versus 1.4 meq g−1). No water/ion clusters were observed in either sample under high humidity or dry conditions. The resulting ion conductivity in the undecyl sample with lamellar morphology was significantly higher in the liquid water saturated state compared to the high humidity state (14.0 mS cm−1versus 4.2 mS cm−1), whereas there was no difference in ion conductivity in the ethyl sample when comparing these two states. These results show that small chemical changes to ion-containing block copolymers can induce water/ion clusters within block copolymer microdomains and this can subsequently have a significant effect on ion transport.

 Please wait while we load your content...
Please wait while we load your content...